Exchange of Radiation
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Heat Transfer
The exchange of radiation is based on two laws. The first, known as Kirchhoff’s law, states that the ratio of the emissive power to the absorptivity is the same for all bodies in thermal equilibrium.
EXCHANGE OF RADIATION
The
exchange of radiation is based on two laws. The first, known as Kirchhoff’s
law, states that the ratio of the emissive power to the absorptivity is the
same for all bodies in thermal equilibrium. The emissive power of a body, E, is
the radiant energy emitted from unit area in unit time (J/m2 sec). A
body of area A1 and emissivity E1, therefore, emits
energy at a rate E1A1. If the radiation falling on unit
area of the body is Eb, the rate of energy absorption is Eba1A1,
where a1 is the absorptivity. At thermal equilibrium, Eba1A1
= E1A. For
another body in the same environment, Eba2A2 = E2A2.
Therefore,
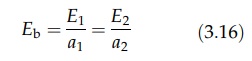
For
a blackbody, a = 1. The emissive power is therefore
Eb. The blackbody is a perfect radiator, and it is used as the
comparative standard for other sur-faces. The emissivity of a surface is
defined as the ratio of the emissive power, E, of the surface to the emissive
power of a blackbody at the same temperature, Eb.
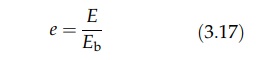
The
emissivity is numerically equal to the absorptivity. Since the emissive power
varies with wavelength, the ratio should be quoted at a particular wavelength.
For many materials, however, the emissive power is a constant fraction of the
blackbody radiation, that is, the emissivity is constant. These materials are
known as gray bodies.
The second fundamental law of radiation, known as the Stefan Boltzmann law, states that the rate of energy emission from a blackbody is proportional to the fourth power of the absolute temperature, T.
E= σT4 (3:18)
where
E is the total emissive power and σ is the Stefan Boltzmann constant, the
numerical value of which is 5.676 x 10-8 J/m2·sec·K4.
It is sufficiently accurate to say that the heat emitted in unit time, Q, from
a blackbody of area A is given by
Q
= σAT4
and
for a body that is not perfectly black by
Q
= σeAT4 (3:19)
where
e is the emissivity.
The
net energy gained or lost by a body can be estimated with these laws. The
simplest case is that of a gray body in black surroundings. These conditions,
in which none of the energy emitted by the body is reflected back, are
approximately those of a body radiating to atmosphere. If the absolute
tem-perature of the body is T1, the rate of heat loss is σeAT14
[equation (3.19)], where A is the area of the body and e is its emissivity.
Surroundings at a temperature T2 will emit radiation proportional to
σT24,
and a fraction, determined by area and absorptivity, a, will be absorbed by the body. This heat will be σaAT24, and
since absorptivity and emissivity are equal,
Net
heat transfer rate =
σeA(T14
- T24) (3:20)
If
part of the energy emitted by a surface is reflected back by another surface,
the calculation of radiation exchange is more complex. Equations for various
surface configurations are available. These take the general form
Q
= F1F2σA(TA4 - TB4)
where
F1 and F2 are factors determined by the configuration and
emissivity of surfaces at temperatures TA and TB.
Example Problems
Example 1
A
stainless steel pipe has an internal radius of 0.019 m and an external radius
of 0.024 m. The thermal conductivity of stainless steel is 34.606 J/m·sec·K.
Steam at 422 K surrounds the pipe that is lagged with 0.051 m of insulation
with a conductivity of 0.069 J/m·sec·K. The temperature of the outer surface of
the insulation is 311 K. What is the heat loss per meter of pipe? For the wall
of the pipe,
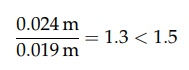
Therefore,
the arithmetic mean best defines the radius.

Therefore,
the logarithmic mean best defines the radius.

For
the insulation,
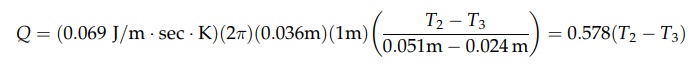
Rearranging
these equations gives the following:
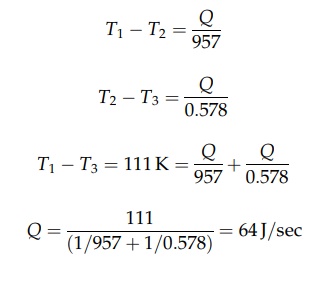
Example 2
A
0.051-m uninsulated horizontal pipe is carrying steam at 389 K to the surroundings
at 294 K. The emissivity, e, of the pipe is 0.8. Absolute zero is 273 K.
Find
the heat loss by radiation.
Heat
loss / Unit length = (5:676 10-8 J=m2 sec K4)(0:058
m)(π)(0:8)[(116K)4 - 21K)4]
= 1.50 J/m sec
Related Topics
