History of WHO Programme - Global Monitoring
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: WHO Programme - Global Monitoring
The Programme was established in 1968 as a pilot project with the participation of 10 countries that had organised national pharmacovigilance systems at that time.
SIGNAL GENERATION
WHO Programme -
Global Monitoring
HISTORY
The
Programme was established in 1968 as a pilot project with the participation of
10 countries that had organised national pharmacovigilance systems at that
time. The intent was to develop international collab-oration to make it easier
to detect rare adverse drug reactions (ADRs) not revealed during clinical
trials. The international drug monitoring centre was moved from the World
Health Organisation (WHO) head-quarters in Geneva, Switzerland, to a WHO
Collab-orating Centre for International Drug Monitoring in Uppsala, Sweden, in
1978. This was the result of an agreement between WHO and the government of
Sweden by which Sweden assumed the operational responsibility for the
Programme. WHO headquarters, Geneva, retained the responsibility for policy
matters. The WHO Collaborating Centre is often referred to as the Uppsala
Monitoring Centre (UMC).
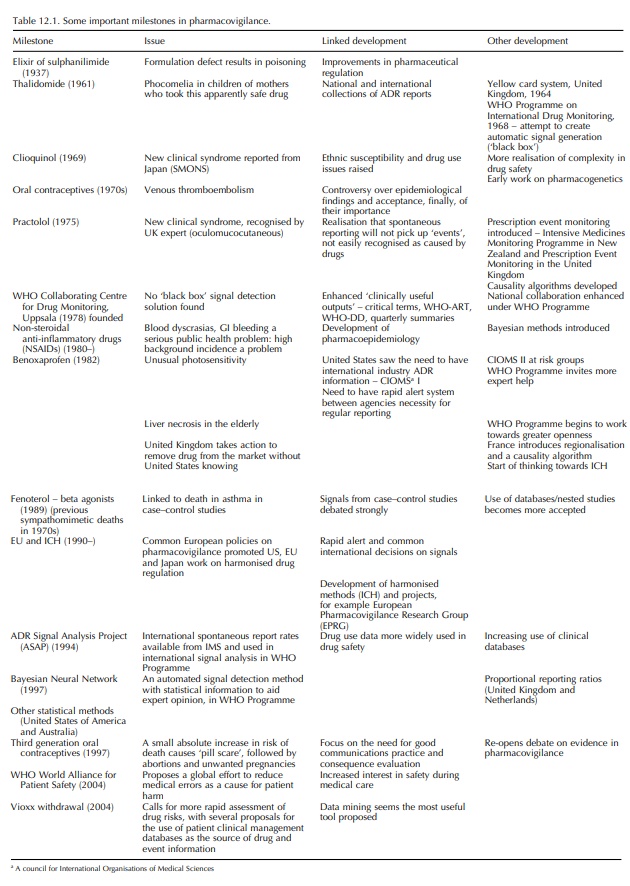
It
is easiest to record the history of pharmacovigilance as a series of milestones
that led to the introduction of new concepts or the re-thinking of old concepts
within the discipline. A chronological list of these milestones is listed in
Table 12.1. It is interesting to note that up to and including the benoxaprofen
(‘Opren’) incident in 1989, changes in drug safety procedures were imple-mented
as a result of drug disasters that had a high media profile. The responses to
these disasters constituted a major re-thinking of drug safety issues. Since
the benoxaprofen incident, there have been many drug withdrawals related to
safety issues, but these have been managed much more effectively and
expeditiously. It may seem that we now have safety systems in place that enable
effective action to be taken globally before disturbing numbers of patients are
affected. However, it is ironic that the pill scare in the United Kingdom may
have caused more distress because of a rapid regulatory response to a safety
issue. Since the benoxaprofen inci-dent, the main changes made in
pharmacovigilance have been proactive improvements involving fine-tuning of
regulatory systems and the adoption of better epidemi-ological techniques often
associated with improve-ments in information technology (IT). Recently, the
withdrawal of the COX2 receptor inhibitor rofecoxib (Vioxx) has led to more
criticism of both the regulatory authorities as well as industry. Chief amongst
these is the slow action taken over the suspicion of an increase in
cardiovascular events. Because this problem is thought to be due to the COX
inhibition, it is very complex because of the variable amounts of COX
selectivity of older NSAIDS as well as many other new drugs with the attribute
of COX2 receptor selectivity. Moreover, there is concern that the COX2 drugs
may not produce the wanted reduction in gastrointestinal bleeding thought to
result from selectivity (Edwards, 2005a).
Related Topics
