Present Programme Structure
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: WHO Programme - Global Monitoring
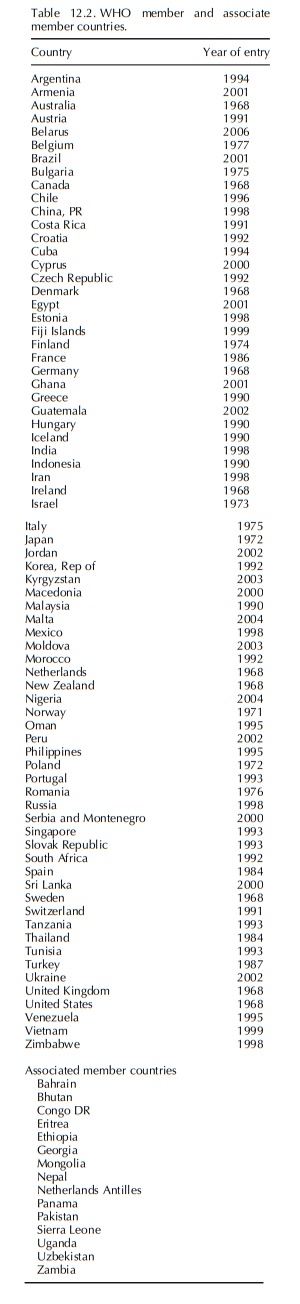
The number of national centres which are active members of the WHO Programme has increased with about three per year during the last few years to the present 78 countries, and the database grows with almost 200 000 reports per year to now over 3.5 million.
PRESENT PROGRAMME STRUCTURE
The
number of national centres which are active members of the WHO Programme has
increased with about three per year during the last few years to the present 78
countries, and the database grows with almost 200 000 reports per year to now
over 3.5 million.
As
pharmacovigilance is developing in many coun-tries in the world, additional
countries continuously formally apply for membership, and they are consid-ered
associate members while the issue of technical compatibility of their reports
with the WHO require-ments is established. Member countries and associate
member countries are listed in the Table 12.2.
In
each country, a national centre, designated by the competent health authority,
is responsible for the collection, processing and evaluation of adverse
reac-tion case reports submitted by health professionals. Information obtained
from these reports is passed back to the professionals on a national basis but
is also submitted to the UMC for inclusion in the WHO international database.
Collectively, the centres annually provide almost 200 000 individual reports to
the WHO of reactions suspected of being drug induced.
Case
reports submitted to the WHO centre accord-ing to an agreed format are checked
for technical correctness and then incorporated in the international database
in a weekly routine. The material is screened four times a year, using Bayesian
Confidence Prop-agation Neural Network (BCPNN) knowledge detec-tion technique
for new and serious reactions. Many additional examinations of the data are
made on an ad hoc basis.
The
WHO Programme global database for ADRs meets or exceeds the ICH E2B agreed
format (http://www.ich.org) and is fully searchable online by the participating
national centres. There is also a web-based software available for reporting
adverse reactions according to the E2B format, called VigiFlow. This software
is used by many for reporting to and between databases independent of WHO.
Related Topics
