Homo-Lumo Interactions
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Planning Organic Syntheses
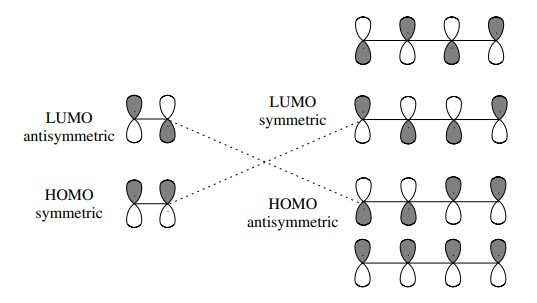
One requirement for a successful HOMO–LUMO interaction is that the symme-try of the HOMO must match the symmetry of the LUMO (either both symmetric or both antisymmetric).
HOMO-LUMO INTERACTIONS
One
requirement for a successful HOMO–LUMO interaction is that the symme-try of the
HOMO must match the symmetry of the LUMO (either both symmetric or both
antisymmetric). If so, then the interaction is symmetry “allowed” and will lead
to productive cycloaddition. If the symmetries do not match, then the HOMO–LUMO
overlap is symmetry “forbidden” and cycloaddition will not pro-ceed. Molecular
orbitals can be classified by their phase symmetry with respect to a plane
normal to the π system. The symmetry
is related to the number of nodal planes which occur in each individual
molecular orbital. For the olefin component, the π orbital (HOMO) is symmetric with respect to this plane and the π ∗ orbital (LUMO) is
antisymmetric with respect to this plane. For the diene component, the HOMO is
antisymmetric and the LUMO is symmetric. Based on these sym-metries, it is seen
that the HOMO–LUMO interaction between butadiene and ethylene is symmetry
allowed and thus can proceed productively to product.
It
turns out that the orbitals for any diene and any olefin have the same symmetry
properties so that all Diels–Alder reactions are symmetry allowed.
While
symmetry requirements dictate whether a cycloaddition can occur, they do not
determine the strength of the HOMO–LUMO interaction. The strength of the
donor–acceptor interaction and the rate of cycloaddition is inversely related
to the difference in energy between the HOMO and LUMO which are interacting. If
the HOMO–LUMO energy gap is small, the interaction is strong and the reac-tion
is rapid, whereas if the HOMO–LUMO energy gap is large, the interaction is weak
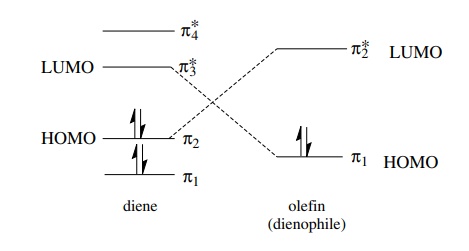
and the reaction is slow. The Diels–Alder reaction between butadiene and
ethylene is very slow, meaning that the donor–acceptor interaction is very weak
because the HOMO–LUMO energy gap is large.
It
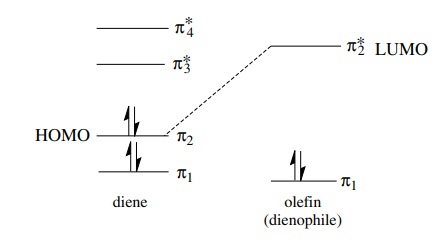
is also pertinent that there are two HOMO–LUMO interactions possible between
butadiene and ethylene, one in which the HOMO is that of the diene, which acts
as the electron donor, and one in which the HOMO is that of the olefin, which
would be the electron donor. A “normal” Diels–Alder reaction is one in which
the diene is electron rich and acts as the electron donor and the olefin
(dienophile) is electron poor and acts as the electron acceptor. In such a case
the diene HOMO and the dienophile LUMO are closer in energy, the donor acceptor
interaction between them is strong, and the reaction takes place at a more
rapid rate.
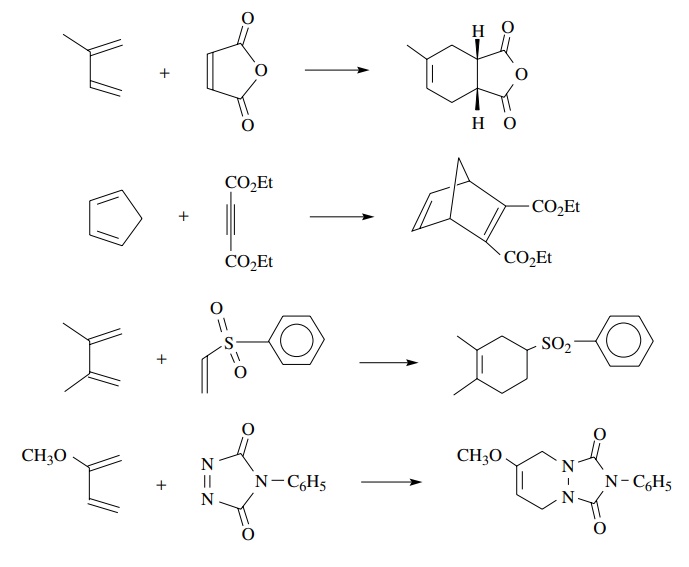
Examples of such partners are dienes substituted with alkyl groups or other electron-donating groups and dienophiles having carbonyl groups attached, which makes them electron deficient. The dienophile need only be a two-electron π system so olefin, acetylene, and azo π systems can all serve effectively as dienophiles so long as they have electron-deficient π bonds. Furthermore the dienophile may be symmetrically or unsymmetrically substituted with electron-withdrawing groups.
Related Topics
