Stereoelectronic Factors
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Planning Organic Syntheses
The interaction between the diene HOMO and the dienophile LUMO takes place when the ends of the two π systems overlap to permit the transfer of electrons from the HOMO into the LUMO.
STEREOELECTRONIC FACTORS
The
interaction between the diene HOMO and the dienophile LUMO takes place when the
ends of the two π systems overlap to
permit the transfer of electrons from the HOMO into the LUMO. This requirement
of overlap imposes stereoelectronic constraints on the two reaction partners.
First, the diene must be able to adopt an s-cis conformation so the ends of the
diene can contact and overlap with the ends of the dienophile π system. For acyclic dienes, even
though the s-trans conformer is favored, rotation about the central
carbon–carbon bond is rapid and there will be a steady-state population of the
required s-cis form present so that the cycloaddition can occur.
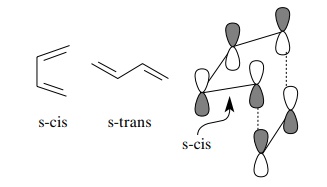
However,
when the diene system is constrained to the s-cis conformation by a cyclic
framework, the effective concentration of the s-cis diene is much higher than
for acyclic dienes, which have the s-cis conformer as a minor component of the
rotomeric equilibrium. Such conformationally constrained dienes react much more
easily and are excellent Diels–Alder dienes. Examples are cyclopentadi-enes,
1,3-cyclohexadienes, and furans.
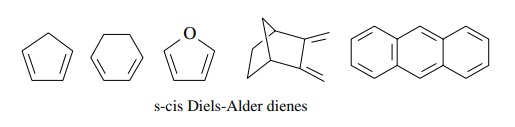
Second,
substituents on the dienophile (olefinic or azo) can adopt a position in the
transition state either exo or endo to the diene system. It has been found that
the endo transition state is favored significantly over the exo transition
state. This preference has been attributed to secondary orbital interactions
(attraction) between the diene and polar substituents on the dienophile.
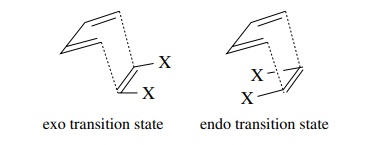
This
distinction is important because exo and endo transition states lead to
different diastereomers. Control of diastereoselection is extremely important
to the utility of the Diels–Alder reaction since mixtures of diastereomers are
avoided and control of multiple stereogenic centers is achieved.
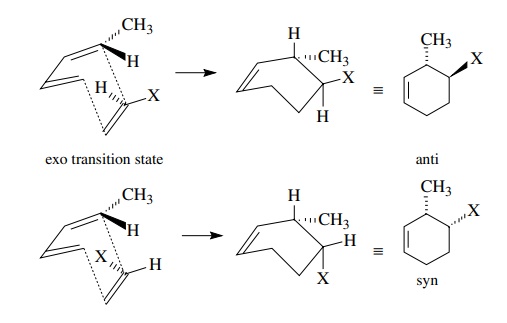
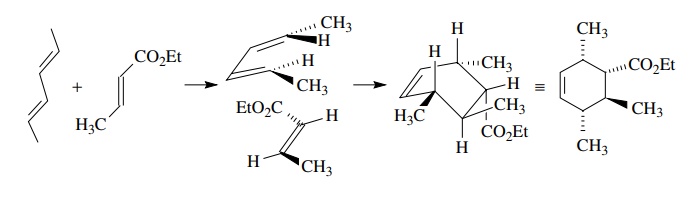
For
example, reaction of (E,E)-2,4-hexadiene with methyl crotonate
gives a sin-gle product in which the relative stereochemistry of four
contiguous stereogenic centers is explicitly defined by the geometry of the
starting materials and the endo transition.
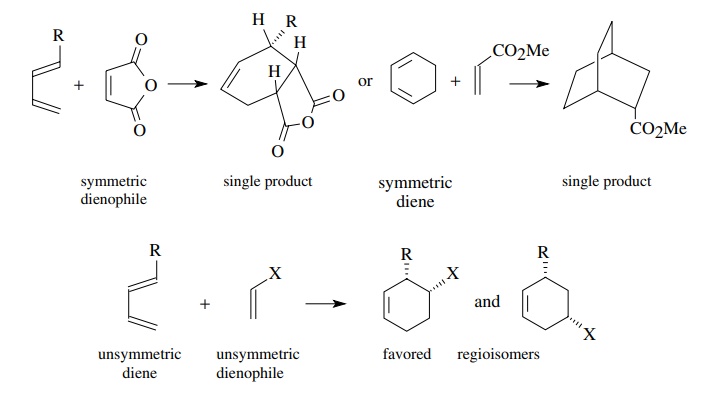
A
third consideration of the Diels–Alder reaction is the regiochemistry of the
products. If either the diene or the dienophile is symmetric, then only a
single regioisomer is possible. If both the diene and the dienophile are
unsymmetric, however, two regioisomers are possible depending on the relative
orientation of the substituents at the transition state. Usually one of these
regioisomers is favored over the other.
The
control of regiochemistry has been rationalized on the basis of the orbital
coefficients of the HOMOs and LUMOs, but in fact, it is not well understood. In
most cases such cycloadditions are not regiospecific and isomeric mixtures are
formed, although one regioisomer usually predominates. Qualitative estimation
of the electron distributions in the diene and dienophile can often be used to
predict the major product. For example, C-1 of siloxy diene (A) should be much more electron rich
than C-4. In addition C-3 of acrylate (B)
should be more electron deficient than C-2.
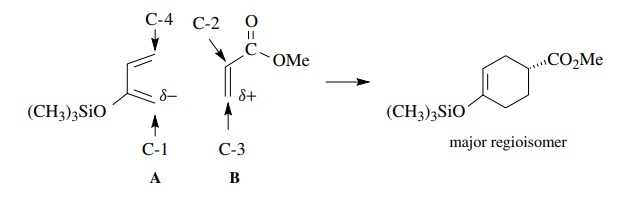
Thus
the most favorable donor–acceptor interaction should occur between C-1 of the
diene and C-3 of the dienophile. This interaction would favor 1,4 orientation
of the substituents in the Diels–Alder product, as is observed.
In
spite of the fact that the major product is often predictable, such systems are
rarely regiospecific. Because mixtures of regioisomers which must be sepa-rated
are the rule, either a symmetric diene or a symmetric dienophile is usually
employed to avoid such regiochemical issues.
The
Diels–Alder reaction can be used to create rings in many situations that would
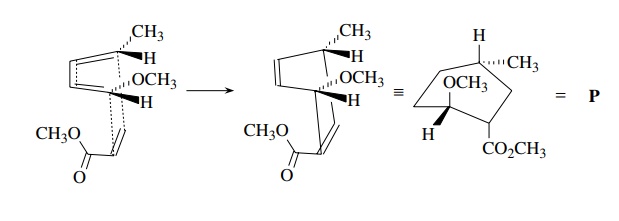
be difficult to accomplish by ring closing approaches. Consider product P. This product can be made by a
Diels–Alder reaction between diene D and acrylate A.
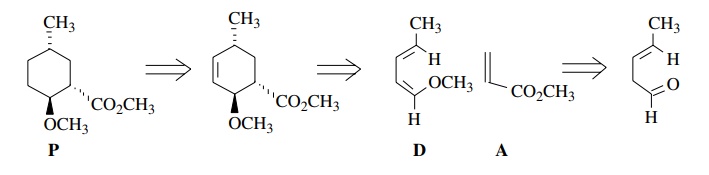
Because
the diene is acyclic, it can achieve the required s-cis conformation by
rotation. The polarity is correct because the diene is electron rich and the
dienophile is electron poor. The stereochemistry is fine because the endo
transition state gives the correct stereochemical relationship of the groups
around the cyclo-hexyl ring. The stereochemistry can be seen more clearly by a
drawing of the transition state.
Related Topics
