Microbial Control by Chemical Methods
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbial Control by Physical and Chemical Methods
A survey of literature would reveal that there exists quite a few well recognized ‘chemical entities’ which are being used in the management and control for the usual growth of microorganisms specifically on both living tissue and inanimate objects.
CHEMICAL
METHODS
A survey
of literature would reveal that there exists quite a few well recognized ‘chemical entities’ which are being
used in the management and control for the usual growth of microorganisms specifically on both living tissue and inanimate objects. However, a relatively much smaller segment of
chemical agents can actually accomplish complete sterility effectively.
Interestingly, a large segment of such substances only succeed either in lowering
the so called ‘microbial populations’
to a much safer levels or getting rid of the vegetative forms of the pathogens** from the infected objects.
As we
have observed under the ‘physical
methods’ that there exists not even a single
appropri-ate method for the effective and meaningful microbial control which may be successfully used in every situation. Exactly, on the same
lines there occurs no one typical
disinfectant which would be perfectly
suitable for most of the prevailing circumstances.
In order
to have a better understanding of the various aspects of the ‘chemical methods of microbial control’, we may extensively
categorize them under the following three heads :
(a) Effective
Disinfection — Fundamentals,
(b) Disinfectant
— Critical Evaluation, and
(c) Variants
— In Disinfectants.
The
aforesaid three classes shall now be
discussed explicity in the sections that follows :
1. Effective Disinfection—Fundamentals
In order
to critically select a disinfectant***
which must serve as an effective agent for complete sterilization one should
bear in mind the following cardinal
factors, namely :
(1) The
concentration of a distinfectant
actually determines its action (which is usually stated on the ‘label’
clearly).
(2) Disinfectant should be diluted strictly
according to the directives given on the ‘label’ by its manufacturer.
(3) Diluted
solutions (very weak) may serve as a bacteriostatic
rather than a bactericidal.
(4) Nature
of the material to be disinfected must be taken into account.
Examples : A few typical examples are :
(a) Organic Substances — may
directly or indirectly interfere with the specific character-istic action of
the disinfectant.
(b) pH — of the medium frequently exerts
a considerable effect upon the disinfectant’s
inherent activity profile.
(5) Accessibility to Microbes. The ease
and convenience with which the
disinfectant is capa-ble of gaining an access to the prevailing microbes
poses a vital consideration. Thus, an area to be treated may require to be scrubbed, and rinsed subsequently just prior to the actual application of the disinfectant. If need be, the disinfectant must be left in contact
with the ‘affected surface’ for many
hours.
(6) Temperature. Higher the temperature used for
the actual application of the ‘disinfectant’,
the higher would be its effectiveness or versatility.
2. Disinfectant—Critical Evaluation
The
critical evaluation of the disinfectants
may be accomplished adopting any one of the fol-lowing two techniques, namely :
(a) Use-Dilution
Tests, and
(b) Filter-Paper
Method.
(a). Use-Dilution Tests
It is,
however, pertinent to state here that there is an absolute necessity to cause
an effective evaluation of the various disinfectants
and antiseptics commonly used.
Phenol-Coefficient Test : It has
been duly employed as the ‘standard
test’, that particularly compared
the activity of a ‘given disinfectant’
with that of ‘phenol’ (as a
standard).
AOAC* Method : The AOAC dilution method is the standard currently being employed for the evaluation of disinfectants. Methodology — Three
strains of microorganisms are usually employed in the AOAC-method, such as : Salmonella
choleraesuis, Staphylococcus aureus, and Pseudomonas aeruginosa. The
various steps involved are as follows :
(1) To
carry out a use-dilution test, the
metal-carrier rings are duly dipped into the standard cultures of the test organism adequately grown in a liquid media—removed carefully– dried at 37°C for a short duration.
(2) Resulting
‘dried cultures’ are subsequently
placed in contact with a solution of the disin-fectant at a concentration
specified by its manufacturer, and left there for a duration of 10 minutes at
20°C.
(3) Consequently,
the carrier rings are duly transferred to a medium which would allow the growth
of any surviving microorganisms.
(4) Result — The actual effectiveness of the
disinfectant may be estimated by the
residual number of cultures.
(b). Filter Paper Method
The filter paper method is commonly used in
the efficacious evaluation of a ‘chemical
agent’ as a disinfectant in
teaching practice in laboratories. A small disk of filter paper (preferably ‘Whatman’ Grade) is duly soaked in a
solution of the ‘chemical agent’,
and placed aseptically on the surface of an agar-plate which has been previously inoculated and incubated duly with a pure test organism. The effectiveness
of the ‘chemical agent’ under
investigation will be exhibited by a clear
zone (known as the zone of
inhibition) designating precisely the inhibition
of growth just around the disk.
3. Disinfectant Variants
A good
number of the disinfectant variants
are being used extensively based on their individual merits and superb
characteristic features, such as :
i.
Alcohols
ii.
Aldehydes,
iii.
Chlorohexidine,
iv.
Gaseous chemosterilizers,
v. Heavy
Metals and Derivatives,
vi.
Halogens,
vii.
Organic Acid and Derivatives,
viii.
Oxidizing Agents,
ix.
Phenol and Phenolics
x.
Quaternary Ammonium Compounds (QUATS), and
xi.
Surface-Active Agents.
The
aforesaid disinfectant variants
shall now be treated individually with appropriate typical examples in the
sections that follows :
i. Alcohols
It has
been duly observed and established that alcohols
specifically exert a bactricidal and
fun-gicidal action quite
effectively. However, they fail to cause any noticeable action upon the endospores and the nonenveloped viruses.
Mechanisms of action : Alcohols
invariably display their activity as a disinfectant due to the protein denaturation of the bacteria.
Besides, they may also cause disinfectant action based on the following two mechanisms, namely :
(a) disruption
of tissue membranes, and
(b) dissolution
of several lipids* (fats).
Advantages : There are as stated under :
(a) They
usually exert their action upon the microbes due to protein
denaturation—evaporating readily—and leaving virtually no residue at all.
(2) Degermination
(or swabbing) of the skin-surface before an injection (IM or IV), the major
component of the microbial control
activity is simply provided by wiping
out the micro-organisms along with the possible presence of the dirt.
Demerit : The main demerit of alcohols as ‘antiseptics’ when applied to the exposed wounds being their ability to cause immediate
coagulation of a layer of protein beneath which the organisms do have a
tendency to grow and multiply.
Examples : The Two most frequently employed
alcohols are, namely :
(1) Ethanol [H5C2–OH]. The usual
recommended optimal strength (concentration) of ethanol is 70% (v/v)
; however, varying concentrations between 60–95% (v/v) appear to cause
bac-tericidal/fungicidal effect quite rapidly. Interestingly, pure ethanol [> 98% (v/v)]
is found to be amazingly less effective in comparison to the corresponding
aqueous ethanolic solu-tions by virtue of the fact that the phenomenon of
denaturation essentially requires water.
(2) Isopropanol [(H3C)2CHOH]
[Syn. : Rubbing Alcohol] — is
observed to be definitely superior to
ethanol as an antiseptic as well as disinfectant. Besides, it is available
more conveniently, less volatile in
nature (than ethanol), and less expensive.
Common Feature : Both ethanol and isopropanol are
remarkably and distinctly employed to augment
(or potentiate) the overall effectiveness of certain other chemical substances.
Examples : Following are two typical examples, namely :
(a) Aqueous Solution of ZephiranTM — is found to kill almost 40% of the
prevailing popula-tion of a ‘test
microbe’ in less than two minutes.
(b) Tincture of ZephiranTM — is observed to kill nearly 85% of the
test organism in just two minutes.
ii. Aldehydes
In
general, the aldehydes are found to
be the most effective antimicrobial
agents (disinfect- ants).
There are two most glaring examples, such as :
(a) Formaldehyde 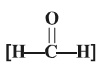 It
invariably causes inactivation of
the proteins by forming the most
critical covalent cross-linkages together with a plethora of ‘organic functional moieties’ on the
proteins viz., —NH2, —OH,
—COOH, and —SH.
It
invariably causes inactivation of
the proteins by forming the most
critical covalent cross-linkages together with a plethora of ‘organic functional moieties’ on the
proteins viz., —NH2, —OH,
—COOH, and —SH.
Important Points — Formaldehyde gas is found
:
(i) to
exert an excellent disinfectant action.
(ii) Formalin (i.e., a 37% aqueous solution of ‘formaldehyde gas’) was previously
employed to embalm dead bodies, to preserve biological specimens, and also to
cause inactivation of microbes and viruses in vaccines.
(b) Glutaraldehyde 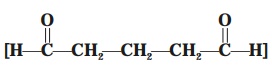 [Syn. : Cidex ; Glutarol, Sonacide ; Verutal ;] — It
represents a chemical entity relative to formaldehyde which being less
irritating and definitely has an
edge over the latter (formaldehyde).
[Syn. : Cidex ; Glutarol, Sonacide ; Verutal ;] — It
represents a chemical entity relative to formaldehyde which being less
irritating and definitely has an
edge over the latter (formaldehyde).
Advantages : These are as given under :
(i) In
the sterilization of various hospital equipments, instruments, including the respiratory-therapy assembly.
(ii) As CidexTM — i.e.,
a 2% (w/v) aqueous solution is usually employed as a bactericidal, virucidal, and tuberculocidal in about 10 minutes ;
whereas as a sporocidal within a
range of 3–10 hours.
(iii) Glutaraldehyde enjoys
the wide-spread recognition and reputation of being the only liquid chemical
disinfectant which may be regarded as a possible sterilant (or sterilizing agent).
iii. Chlorohexidine
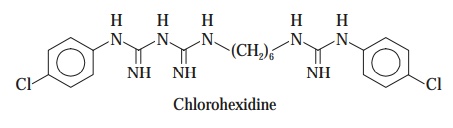
Obviously,
chlorohexidine is not a phenol but its chemical struc
ture and uses are very much identical to those of hexachlorophene.

It is
abundantly used for the disinfection of mucous membranes as well as skin
surfaces.
Merits :
An
admixture with either alcohol (H5C2–OH)
or detergent (surface-active agent)
its usage has been justifiably extended to surgical
hand scrubs and in such patients requiring pre-operative skin preparations.
Mechanism : The probable mechanism of action
of chlorhexidine are as follows :
(a) due
to its distinctly strong affinity for getting adequately bound either to the skin or mucous membranes, thereby
producing its low toxicity.
(b) its cidal effect (i.e., killing effect) is virtually related to the actual damage it
renders to the plasma membrane.
Advantages—Chlorhexidine is found to be advantageous in two particular instances, namely :
(i) Effective
against most vegetative microorganisms,
but certainly is not sporicidal in
nature, and
(ii) Certain
enveloped (i.e., lipophilic) types
of viruses are affected exclusively.
iv. Gaseous Chemosterilizers
Gaseous chemosterilizers may be
defined as—‘chemicals that specifi cally
sterilize in a closed environment.’*
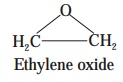
Example : The typical example being Ethylene oxide.
Mechanism : The most probable mechanism of
action of ethylene oxide solely
depends upon its inherent ability to
cause ‘denaturation of proteins’. In
fact, the labile H-atoms strategically
attached to the proteins viz., —OH,
—SH, or —COOH are critically replaced by the available alkyl moieties (alkylation), for instance : —H2C—CH2—OH.
Advantages—These are as stated below :
(1) Ethylene oxide practically
kills all microorganisms besides the endospores ; however, it may require a perceptionally lengthy
exposure ranging between 4–18 hours.*
(2) It
has an extremely high degree of penetrating power to such an extent that it was
specifically selected for the complete sterilization
of spacecraft despached to land on the Moon
plus certain other planets.
5. Heavy Metals and Derivatives
A
plethora of heavy metals and their corresponding derivatives viz., Hg, HgCl2, Cu, CuSO4,
Ag, AgNO3, Zn, ZnCl2 find extensive usages as germicidal and antiseptic agents.
Mechanism — Oligodynamic action refers to the precise ability of relatively
smaller quantum of heavy metals viz., Ag and Cu, to predominantly exert
antimicrobial activity. In reality, the respective metal ions (e.g., Ag+ and Cu2+)
categorically combine with the specific—SH moieties critically located on the ‘cellular proteins’ thereby causing denaturation ultimately.
Examples : A few typical examples are cited
below :
(a) Ag in AgNO3 1% (w/v) Solution : It was a mandatory
practice earlier to treat the eyes of the newborns with a few drops of silver
nitrate solution to prevent and protect against any possi-ble infection of the
eyes usually termed as gonorrheal
ophthalmia neonatorum.**
(b) HgCl2 : It perhaps enjoy the longest
historical usage as an effective disinfectant.
It indeed possessed a rather broad-spectrum of activity together with its prime bacteriostatic activ-ity. The
usage of the ‘mercurochrome antiseptic’ (i.e., an organic mercury compound) is still prevalent in the domain of domestic chests.
(c) CuSO4 : It finds its abundant utility for the
critical destruction of green algae
(an algicide) which grow profusely
in fish-aquariums, swimming pools,
and reservoirs.
(d) ZnCl2 : It is mostly an essential ingredient
in mouth washes like ‘Listerine’
etc. It also serves as a potential antifungal
agent in acrylic-based paints.
vi. Halogens
The two most important halogens that are effectively employed as the antimicrobial agents are iodine
(I2) and chlorine (Cl2)
frequently in solution ; besides, being used as the integral constituents
of both organic or inorganic
compounds.
(a) Iodine (I2) : The most
commonly used Iodine Solution was
the Iodine Tincture*, which has
become more or less obsolete nowadays ; and has been duly replaced by Iodophor.
An iodophor may be defined as — ‘an unique combination of iodine and an
organic molecule, from which iodine gets released gradually’.
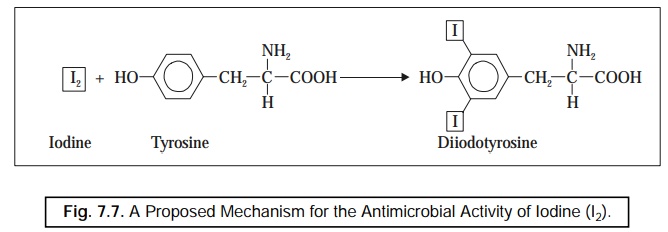
Mechanism : The most probable and proposed
mechanism for the activity of iodine being that it particularly and critically
gets combined with tyrosine–an amino acid which essen tially represents
an integral common constituent of :
·
several enzymes, and
·
many cellular proteins,
as
depicted in Figure 7.7.
Advantages of an Iodophor : It
essentially possesses three major advantages, namely
:
·
Possesses the same activity as that of iodine as an antimicrobial agent,
·
Does not stain either the skin surface or clothes,
and
·
It is much less irritating in nature (contrary to
the iodine tincture).
Example : The most typical example is that
of :
Povidone Iodines [Syn. : Betadine(R) ; Isodine(R)] which
essentially improves the wetting action due to the fact
that povidone is a surface-active
iodophor.
Uses : Iodines are used exclusively for
the treatment of infected wounds and skin infec-tions.
Note : However, the Pseudomonas may adequately survive for comparatively longer
durations in iodophores.
(b) Chlorine (Cl2) : As to
date chlorine (Cl2) finds
its abundant use as a disinfectant in the form of a ‘gas’ or in combination with certain other chemical substances.
Mechanism : The probable mechanism whereby chlorine exerts its germicidal action is on account of the production of hypochlorous acid (HOCl) which forms
specifically on the incorporation of chlorine
to water. The various chemical reactions which take place may be expressed
as under :
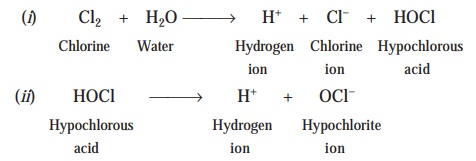
Hypochlorous Acid. The
precise and exact mechanism whereby
hypochlorous acid causes the ‘cidal
effect’ (i.e., killing power) is not yet known fully.
Neverthless, it is indeed a strong
oxidizing agent which eventually blocks and prevents a major segment of the vital cellular enzyme system to function
in a normal manner.
Advantages : There are two main advantageous functionalities of hypochlorous acid, namely :
(a) It
represents the most effective form of chlorine
(Cl2) by virtue of it being absolutely neutral with respect to
its electrical charge ; and, therefore, undergoes diffusion as quickly as
possible via the cell wall.
(b) The hypochlorite ion [OCl–] [see
Eqn. (ii)] bears a distinct negative
charge which critically renders its free entry and access into the body of the infected cell.
Liquid Chlorine Gas : The usage
of pure liquid form of compressed chlorine (Cl2) gas is invari-ably
done for carrying out the effective disinfection of municipal supply of potable (drinking) water, swimming-pool water, and
sometimes even the municipal sewage-drain outlets.
Compounds of Chlorine : A good
number of compounds of chlorine viz., calcium hypochlorite [Ca(OCl)2], and sodium hypochlorite [NaOCl] are
largely employed as effective
disinfectants.
Ca(OCl)2 is used
to disinfect both the ‘dairy-equipments’
and ‘cooking/eating utencils’ in eateries (restaurants).
Clorox(R). It is a
frequently used household disinfectant and
a bleach that finds its extensive applications in various industrial and
hospital environments, such as :
Dairy-Processing Organisations —
industry ;
Food-Processing Establishments —
industry ; and
Haemodialysis Systems — hospital.
vii. Organic Acids and Derivatives
A large
number of organic acids are employed
both extensively and profusely as potential pre-servatives to control the
growth of mold.
Examples : There are several typical
examples, such as :
(a) Benzoic Acid [or salt derivative Sodium
Benzoate] is duly recog nized as a vital antifungal
agent which is observed to be extremely effective at relatively lower pH
values.
Uses : Benzoic acid/Sodium benzoate are
employed extensively in a broad range
of acidic food products viz., pickles, lime juices ; bev erages viz., soft drinks, lime
cordials, fruit squashes, canned fruit-juices ; and processed food products viz., fruit jams, cheese, neat products,
vegetables/fruits (canned), tomatopastes,
tomato-sauces, and the like.
(b) Sorbic Acid [or salt derivative Potassium
Sorbate] is invariably employed to prevent and inhibit the mold growth in acidic foods
particularly viz., cheese.

(c) Parabens — e.g., methylparaben and propylparaben find their abundant
applications to control and inhibit mold
growth in galenicals, liquid
cosmetics, foods, shampoos, and beverages.
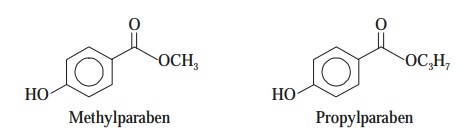
[Note : Parabens are nothing but derivatives of
‘benzoic acid’ that essentially work at a neutral pH (viz., 7).]
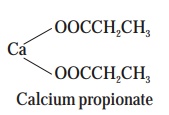
(d) Calcium Propionate— is an
inhibitor of moulds and other microor ganisms invariably found in a
wide-spectrum of products, such as :
foods,
tobacco, pharmaceuticals, butyl-rubber to improve the processability, and
scorching resistance.
Mechanism : The precise mechanism of activity
of these aforesaid organic acid and their
respective derivatives is not exclusively associated to their inherent acidity but realistically to the
following two cardinal aspects,
namely :
(i) inhibition
of enzymatic activity, and
(ii) inhibition
of metabolic activity.
In a
rather broader perspective the human body is capable of metabolizing these organic acids quite rapidly thereby rendering
their usage in vivo quite safe in all respects.
viii. Oxidizing Agents
It has
been observed that the oxidizing agents usually display and exert their ‘antimicrobial activity’ by specifically oxidizing the cellular components of the
treated microorganisms.
A few
typical examples are discussed briefly as under :
(a) Ozone [O3]. It is an
extremely reactive state of oxygen (O2) that may be generated by
pass-ing oxygen via a high-voltage
electrical discharge system. In fact, one may critically observe the presence
of ozone in the following particular
instances :
·
presence of air’s fresh odour immediately after a
lightning storm,
·
nearest place to a reasonably large electric spark,
and
·
in the vicinity of an UV light (or lamp).
Important Points : There are two vital points to note :
(i) Though
ozone [O3] exerts a more
effective, marked and pronounced cidal
effect (or killing effect), yet
its overall residual activity is practically difficult to main-tain in water,
and
(ii) Ozone is definitely more expensive
than chlorine as an antimicrobial agent.
(b) Hydrogen Peroxide [H2O2]
: Hydrogen peroxide finds a pivotal place in several hospital supply
facilities as well as household medicine cabinets.
Mechanism : Ozone gets
rapidly cleaved into water and nescent gaseous oxygen due to the critical action of the enzyme catalase usually found in human cells,
as illustrated under :
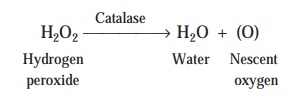
Perhaps
it could be the valid supportive evidence and proof that ozone fails to serve as a ‘good
antiseptic’ particularly for the
open wounds.
Uses : These are as follows :
(1) It
effectively disinfects the inanimate
(i.e., showning no sign of being
alive) objects.
(2) It
proves to be sporocidal in nature,
specifically at elevated temperature(s).
(3) Presence
of usual protective enzymes belonging
to the aerobic microorganims, and
the facultative anaerobes in the non-living surface zones, are found to
be largely overwhelmed by the critical
high concentrations of hydrogen peroxide actually em-ployed.
Based on
these stark realities and superb functionalities the hydrogen peroxide is frequently used in
:
·
food industry for ‘aspectic packaging’,* and
·
users of ‘contact
lenses’ (i.e., a pharmaceutical aid) usually disinfect
them (lenses) with H2O2. After carrying out the said
disinfection procedure, a Pt-catalyst
invariably present in the lens-disinfecting
kit helps to cause destruction of the residual
H2O2 ;
and, therefore, it no more persists on the contact
lens, where it could serve as an irritant.
(c) Benzoyl Peroxide [Syns. : Debroxide
; Lucidol ; Nericur ; Sanoxit ;
Theraderm ; Xerac BP ;] — Benzoyl
peroxide is an useful oxidizing agent for treating such wounds
that are usually infected by the anaerobic
pathogens. However, it is found to be the major component in most of the
over-the-counter (OTC) medi-caments meant for curing acne** that is generally caused by a specific kind of anaerobic bacterium infecting the hair-follicles.
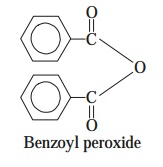
ix. Phenol and Phenolics
Phenol [Syn.
: Carbolic acid ; Phenic acid ;] happens to be the first and
foremost chemical substance that was
duly used by the famous British Physician Joseph Lister for sterilization of
his ‘op-eration theater’. However,
it has become quite obsolete as an antiseptic or disinfectant due to two major drawbacks, namely :
·
irritating action on skin, and
·
highly inherent sharp disagreable odour.
Phenolics i.e., derivatives of phenol, which
essentially contain a phenolic moiety that has been meticulously and chemically modified to accomplish the following two important objectives :
(a) in
minimizing phenol’s most irritating qualities, and
(b) in
enhancing phenol’s antimicrobial activity in combination with either a detergent or a soap.
Mechanism — Phenolics predominantly
exert its antibacterial activity by
injuring the plasma membranes particularly ; besides, denaturation of proteins, and
inactivation of enzymes.
Uses : The various uses of phenolics are
as stated under :
(a) As
disinfectants due to the fact that they usually remain active even in the presence
of organic compounds.
(2) Phenolics are found to be fairly stable in
nature.
(3) Phenolics do persist for a relatively
longer duration of action after their adequate treat-ment.
(4) Phenolics find their abundant usage as the
most sort after and adequately suitable anti-microbial agents particularly for
the disinfection of saliva, pus, and
faeces.
Examples : There are two most important and typical examples of phenolics, such as :
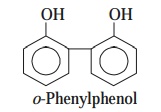
(a) o-Phenylphenol
[Syn. : Orthoxenol ; Dowicide ;] : It is an
extremely important cresol
originally derived from a group of coal-tar chemi-cals. In fact, o-phenylphenol
constitute as the major ingredient in most formulations of Lysol(R). Generally, the cresol do serve as very good
surface disinfectants.
(b) Hexachlorophene [Syn. : Bilevon ;
Dermadex ; Exofene ; Hexosan ; pHisohex ; Surgi-Cen ; Surofene ;] :
Hexachlorophene was initially used abundantly as
a vital constituent in a host of antiseptic, cosmetic, and allied for
mulations, such as : surgical scrubs, cosmetic soaps, deodor ants, feminine
hygiene sprays, toothpastes, and hospital bac terial control procedures.
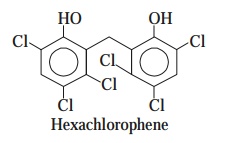
It is
found to be effective as a bacteriostatic
agent, and specifically effective against two Gram-positive organisms viz.,
Staphylococci and Streptococci which usually cause
dermatological infec-tions.
Note : US-FDA, in 1972, has regulated the use of
hexachlorophene because of its potential neurotoxicity in humans.
Uses :
(1) Hexachlorophene is
chiefly used in the manufacture of the germicidal soaps.
(2) It is
a potential antiseptic and disinfectant.
x. Quaternary Ammonium Compounds [QUATS]
It has
been established beyond any reasonable doubt that the most profusely employed surface-active agents are essentially
the cationic detergents, and
particularly the quaternary ammonium
compounds [QUATS]. Importantly,
the highly effective and the most potential cleansing ability solely resides to the positively charged segment—the cation of the molecular entity.
Nevertheless,
the quaternary ammonium compounds
are observed to be strongly
bacte-ricidal against the Gram-positive microorganisms, and
apparently reduced activity profile against
the Gram-negative microorganisms.
QUATS—are found to be amoebicidal, fungicidal, and virucidal against
the enveloped viruses particularly.
QUATS—fail to exert cidal effect on the endospores or tuberculosis organism i.e., Mycobacterium
tuberculosis.
Mechanism—The exact chemical mode of action of
QUATS are not known explicitely ; however,
they most probably do affect the plasma membrane particularly. Noticeable
change in the cell’s permeation ability may be seen thereby resulting into the
appreciable quantum loss of the most vital ‘cytoplasmic
components’ e.g., potassium.
Examples : There are two quite common and widely popular QUATS, such as :
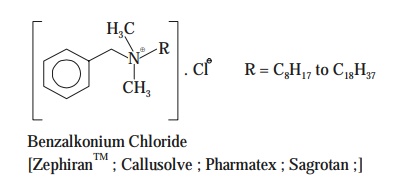
(a) Benzalkonium chloride—[i.e., ZephiranTM—the brand name],
(b) Cetylpyridinium chloride—[i.e., Cepacol(R)—the brand name].
The
following Figure : 7.8 clearly depicts the ammonium
ion vis-a-vis quaternary ammonium compounds viz., Benzalkonium chloride
[ZephiranTM], and Cetylpyridinium chloride [Cepacol(R)].
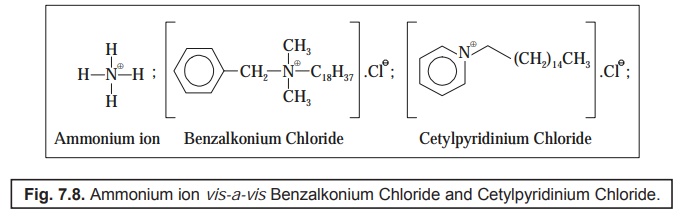
From Fig.
7.8 one may evidently observe the manner whereby the other moieties
strategically replace the hydrogen atoms of the ammonium ion.
Interestingly,
both the above cited QUATS are found
to be absolutely colourless, odourless, tasteless, fairly stable, easily
diluted, nontoxic in nature, possess strongly antibacterial activities—ex-cept
at relatively high concentrations.
Salient Features—The salient features of these QUATS are as stated under :
(1) Presence
of ‘organic matter’ squarely
interferes with the activities of QUATS.
(2) They
are neutralized almost instantly on coming in contact with either the anionic deter-gents or the soaps.
(3) Pseudomonas
do
survive in the presence of QUATS, and subsequently grow in them.
(4) Broadly
recognized as pharmaceutic aid
(preservative).
xi. Surface-Active Agents [or Surfactants]
Surface-active agents may be defined as—‘substances that specifically lower, the
surface tension prevailing amongst the molecules of a liquid. Such agents
essentially include oil, soaps, and various types of detergents.
Soap—The soap is made by the
saponification of vegetable oils with the removal of glycerine as a by-product. Though it possesses
rather little value as an antiseptic/disinfectant as such, but it does exert an
extremely important function in the mechanical removal of microorganisms by
means of gentle scrubbing*.
In actual
practice, the soap actually aids in the careful cleavage of the thin-oily film
(present on the skin-surface) via a
superb phenomenon invariably termed as emulsification,
whereby the mixture of water/soap meticulously abstracts the emulsified oil
together with the debris of dead cells,
dirt particulate matters, and microorganisms, and float them away
swiftly when the latter thus produced is flushed out with water.
Uses :
(1) In
general, soaps do serve as reasonably good and efficacious degerming agents.
(2) Deodorant soap essentially
containing typical chemical entities e.g., triclocarban, predomi-nantly inhibit the Gram-positive microorganisms.
Triclocarban [Syn : Cutisan ; Nobacter ;
Solubacter ;] :
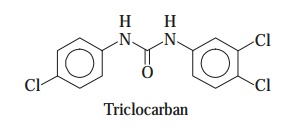
Triclocarban finds its abundant usage as a bacteriostat and antiseptic in soaps (medicated) and other cleansing compositions.
Acid-Anionic Surface-Active Sanitizers : They
usually designate an extremely vital and im-portant group of chemical
substances that are being used extensively in the cleaning of dairy utensils
and equipments. It has been duly observed that their ‘sanitizing ability’ is duly confined to the strategic negatively charged
segment (anion) of the molecule, that eventually interacts critically with the
re-spective plasma membrane. Besides, such type of sanitizers invariably exert
their action upon a broad spectrum of the microorganisms, even including
certain most fussy and troublesome thermoduric
mi-crobes. In reality, these sanitizers are found to be absolutely
nontoxic, fast-acting, and above all noncorrosive
in nature.
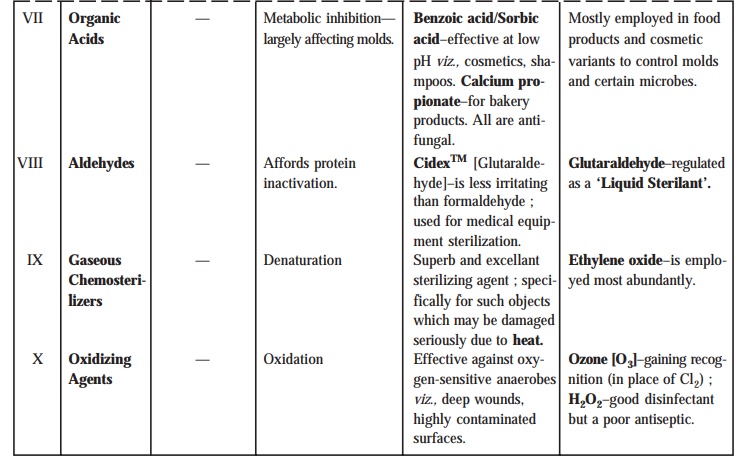
Table :
7.4 records a summarized details of the various chemical agents, as described from Sec-tions 7.3.3.1 to 7.3.3.11,
that efficiently controls the microbial
growth in general.

Related Topics
