Methods of Sterility Testing: Pharmaceutical Products
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Sterility Testing: Pharmaceutical Products
In a broader perspective the wide-spectrum of the pharmaceutical products, both pure and dosage forms, may be accomplished by adopting any one of the following two well-recognized, time-tested, and universally accepted methods, namely : (a) Membrane Filtration, and (b) Direct Inoculation.
TEST FOR
STERILITY : PHARMACEUTICAL PRODUCTS
In a
broader perspective the wide-spectrum of the pharmaceutical products, both pure and dosage forms, may be
accomplished by adopting any one of the following two well-recognized, time-tested, and universally accepted methods,
namely :
(a) Membrane
Filtration, and
(b) Direct
Inoculation.
These two methods stated above shall now be
treated individually in the sections that follows :
1. Membrane Filtration
The membrane filtration method has gained
and maintained its glorious traditional recognition to not only circumvent but
also to overcome the activity of
antibiotics for which there exist practically little inactivating agents. However, it may be duly extended to embrace
legitimately a host of other relevant products as and when deemed fit.
Importantly,
the method emphatically requires the following characteristic features, namely
:
·
an exceptional skill,
·
an in-depth specific knowledge, and
·
rigorous routine usage of positive and negative
controls.
As a
typical example of a suitable positive
control with respect to the appropriate usage of a known ‘contaminated solution’ essentially
comprising of a few microorganisms of altogether different nature and types*.
Salient Features : The salient features of the ‘membrane filtration’ method are as
enumerated under :
(1) The
solution of the product under investigation is carefully filtered via a hydrophobic-edged membrane
filter that would precisely retain any
possible contaminating microorganisms.
(2) The
resulting membrane is duly washed in situ
to get rid of any possible ‘traces of
antibiotic’ that would have been sticking to the surface of the membrane
intimately.
(3) Finally,
the segregated microorganisms are
meticulously transferred to the suitable
culture media under perfect
aseptic environment.
Microorganisms for Positive Control Tests : There
are, infact, four typical microorganisms that
are being used exclusively for the positive
control tests along with their respective type of specific enzymatic
activity mentioned in parentheses :
(a) Bacillus cerreus : [Broad
spectrum] ;
(b) Staphylococcus aureus :
[Penicillinase] ;
(c) Klebsiella aerogenes :
[Penicillinase + Cephalosporinase] ; and
(d) Enterobacter species :
[Cephalosporinase].
Interestingly,
the microorganisms invariably employed for the positive control tests together with a particular product
containing essentially an ‘antimicrobial
agent’ must be, as far as possible, explicitely
sensitive to that agent, in order that the ultimate growth of the microbe solely indicates three
vital and important informations, namely :
·
satisfactory inactivation,
·
satisfactory dilution,
and
·
satisfactory removal
of the agent.
Specific Instances of Pharmaceutical Products : Virtually
all the ‘Official Compendia’ viz., Indian Pharmacopoea (IP) ; British Pharmacopoea (BP), United States
Pharmacopoea (USP) ; European Pharmacopoea (Eur. P), and International Pharmacopoea (Int. P.) have
duly provided comprehensive and
specific details with regard to the ‘tests
for sterility’ of parenteral products
(e.g.., IV and IM injectables), ophthalmic preparations (e.g., eye-drops, eye-ointments,
eye-lotions etc.) ; besides a plethora of non-injectable
preparations, such as : catgut,
dusting powder, and surgical
dressings.
Test Procedures : In a
broader perspective, the membrane
filtration is to be preferred exclu-sively in such instances where the
substance under investigation could any one of the following four classes of pharmaceutical preparations :
(i) an oil or oil-based product,
(ii) an ointment that may be put into solution,
(iii) a non-bacteriostatic solid that does not
become soluble in the culture medium rapidly, and
(iv) a soluble powder or a liquid that essentially possesses
either inherent bacteriostatic or inherent fungistatic characteristic
features.
The membrane filtration must be used for
such products where the volume in a container is either 100 mL or more. One
may, however, select the exact number of samples
to be tested from Table 8.1 ; and subsequently use them for the respective culture
medium suitably selected for microorganisms
and the culture medium appropriately selected for fungi.
Precautionary Measures : In actual
practice, however, the tests for
sterility must always be carried
out under highly specific experimental parameters so as to avoid any least
possible accidental contamination of the product being
examined, such as :
(a) a
sophisticated laminar sterile airflow
cabinet (provided with effective hepa-filters),
(b) necessary
precautionary measures taken to be such so as to avoid contamination that they
do not affect any microbes which must be revealed duly in the test.
(c) ensuing
environment (i.e., working
conditions) of the laboratory where the ‘tests
for sterility’ is performed must always be monitored at a definite
periodical interval by :
·
sampling the air
of the working area,
·
sampling the surface
of the working area, and
·
perforing the stipulated control tests.
Methodology : In usual practice, it is
absolutely urgent and necessary to first clean meticulously the exterior surface of
ampoules, and closures of vials and
bottles with an appropriate antimicrobial
agent ; and thereafter, the actual
access to the contents should be gained carefully in a perfect aseptic manner.
However, in a situation where the
contents are duly packed in a particular container under vacuum, introduction
of ‘sterile air’ must be done by the
help of a suitable sterile device, for instance : a needle duly attached to a syringe barrel with a non-absorbent cotton.
Apparatus : The most suitable unit comprises
of a closed reservoir and a receptacle between which a properly supported membrane
of appropriate porosity is placed strategically.
·
A membrane usually found to be quite suitable for
sterility testing essentially bears a nominal
pore size not more than 0.45 μm, and diameter of nearly 47 mm, the
effectiveness of which in the
retention of microbes has been established adequately.
·
The entire unit is most preferably assembled and
sterilised with the membrane in place prior to use.
·
In case, the sample happens to be an oil, sterilize the membrane separately
and, after thorough drying, assemble the unit, adopting appropriate aseptic
precautionary measures.
Diluting of Fluids : In the ‘test for sterility’ one invariably
comes across with two
different types of fluids which
will be treated individually in the sections that follows :
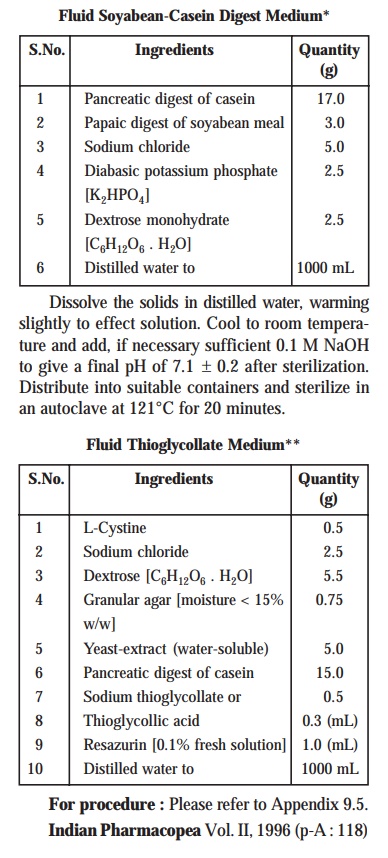
(a) Fluid A—Digest 1 g of peptic digest of animal tissue* or its
equivalent in water to make up the volume upto 1L, filter or centrifuge to
clarify, adjust to pH 7.1 ± 0.2,
dispense into flasks in 100 mL quantities, and finally sterilize at 121° C for
20 minutes (in an ‘Autoclave’).
Note : In a specific instance, where Fluid A is to
be used in carrying out the tests for sterility on a specimen of the penicillin
or cephalosporin class of anibiotics, aseptically incorporate an amount of
sterile penicillinase to the Fluid A to be employed to rinse the membrane(s)
sufficient to inactivate any residual antibiotic activity on the membrane(s)
after the solution of the specimen has been duly filtered.
(b) Fluid B : In a specific instance, when the
test sample usually contains either oil
or lecithin*, use Fluid A to each litre of which has been
added 1 mL of Polysorbate 80**,
adjust to pH 7.1 ± 0.2, dispense into flasks and sterilize at 121° C for 20
minutes (in an ‘Autoclave’).
Note : A sterile fluid shall not have either
antimicrobial or antifungal properteis if it is to be considered suitable for
dissolving, diluting or rinsing a preparation being examined for sterility.
Quantum of Sample Used for ‘Tests for Sterility’ : In fact,
the exact and precise quantities of sample
to be used for determining the ‘Tests
for Sterility’ are quite different for the injectables and ophthalmics plus
other non-injectables ; and,
therefore, they would be discussed separately as under :
(a) For Injectable Preparations : As a
common routine practice and wherever possible always use the whole contents of
the container ; however, in any case not less than the quantities duly stated
in Table : 8.2, diluting wherever necessary to 100 mL with an appropriate sterile diluent e.g., Fluid A.
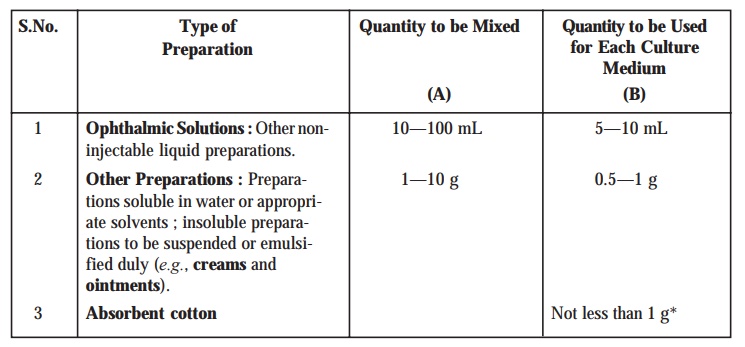
(b) For Ophthalmic and other Non-injectable
Preparations : In this particular instance exactly take an amount
lying very much within the range prescribed in Column (A) of Table : 8.3, if
necessary, making use of the contents of more than one container, and mix
thoroughly. For each specific medium use the amount duly specified in column
(B) of Table : 8.3. taken carefully from the mixed sample.
Table : 8.2. Quantities of Liquids/Solids per
Container of Injectables Vs Minimum
Quantitiy Recommended for Each Culture Medium.
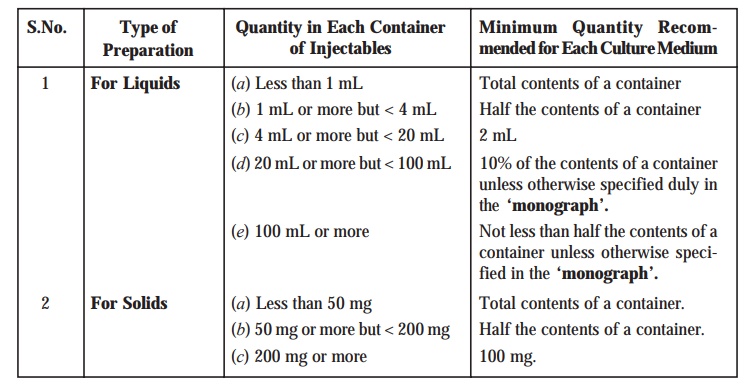
Table : 8.3. Type of Preparation Vs Quantity to be Mixed and Quantity to
be Used for Each Culture Medium
Method of Actual Test : In
reality, the method of actual test may
be sub-divided into the following four categories, namely :
(i) Aqueous
Solutions,
(ii) Liquids
Immiscible with Aqueous Vehicles and Suspensions
(iii) Oils
and Oily Solutions, and
(iv) Ointments
and Creams.
These three aforesaid types of pharmaceutical
preparations shall be treated separately as under :
[I] Aqueous Solutions : The
following steps may be followed sequentially :
(1) Prepare
each membrane by transferring aseptically a small amount (i.e., just
sufficient to get the membrane moistened duly) of fluid A on to the membrane and filtering it carefully.
(2) For
each medium to be employed, transfer aseptically into two separate membrane filter funnels
or two separate sterile pooling
vessels prior to transfer not less than the quantity of the prepa-ration
being examined which is duly prescribed either in Table : 8.2 or Table : 8.3.
(3) Alternatively,
transfer aseptically the combined quantities of the preparation being examined
prescribed explicitely in the two
media onto one membrane exclusively.
(4) Suck
in the ‘liquid’ quickly via the membrane filter with the help of a negative pressure (i.e., under vacuum).
(5) In
case, the solution being examined has significant
antibacterial characteristic features, wash the membrane(s) by filtering
through it (them) not less than three
successive quantities, each of approximately 100 mL of the sterile fluid A.
(6) Precisely,
the quantities of fluid actually employed must be sufficient to permit the
adequate growth of a ‘small inoculum of
microorganisms’ (nearly 50) sensitive to the antimicrobial substance in the
presence of the residual inhibitory material retained duly on the membrane.
(7) Once
the filtration is completed, aseptically remove the membrane(s) from the
holder, cut the membrane in half, if only one is used, immerse the membrane or
1/2 of the membrane, in 100 mL of the ‘Fluid
Soyabean-Casein Digest Medium’*, and
incubate at 20–25°C for a duration of seven days.
(8) Likewise,
carefully immerse the other membrane, or other half of the membrane, in 100 mL
of ‘Fluid Thioglycollate Medium’,**
and incubate duly at 30–35° C for not
less than seven days.
[II] Liquids Immiscible with Aqueous Vehicles and
Suspensions : For this one may carry out the ‘test’ as
stipulated under [I] Aqueous Solutions,
but add a sufficient amount of fluid A
to the pooled sample to accomplish fast and rapid rate of filtration.
Special Features : These are
as stated under :
(1) Sterile enzyme preparations, for
instance :
Penicillinase
Cellulase
can be
incorporated to fluid A to help in
the dissolution of insoluble substances.
(2) In a
situation when the substance under test usually contains lecithin, alway make use of fluid
B for dilution.]
[III] Oils and Oily Solutions : The
various steps that are essentially involved in treating oils and oily solutions for carrying out the ‘test for sterility’ are as enumerated under :
(1) Filter
oils or oily solutions of
sufficiently low vicosity as such i.e., without any dilution via a dry membrane.
(2) It is
absolutely necessary to dilute viscous oils as necessary with an appropriate sterile diluent e.g., isopropyl myristate which
has been proved beyond any reasonable doubt not to exhibit any antimicrobial
activities under the prevailing parameters of the test.
(3) Permit
the ‘oil’ to penetrate the membrane,
and carry out the filtration by the application of gradual suction (with a
vaccum pump).
(4) Wash
the membrane by filtering through it at least 3/4 successive quantities, each
of nearly 100 mL of sterile fluid B
or any other appropriate sterile diluent.
(5) Complete
the test as described under [I] Aqueous
Solutions from step (7) onwards.
[IV] Ointments and Creams : The
various steps involved are as stated under :
(1) Dilute
ointments carefully either in a ‘fatty base’ or ‘emulsions’ of the water-in-oil
(i.e., w/o) type to yield a fluid concentration of approx. 1% w/v, by
applying gentle heat, if necessary, to
not more than 40°C with the aid of an appropriate sterile diluent e.g., isopropyl myristate previously adequately sterilized by filtration via a 0.22 μm
membrane filter which has been shown not to possess antimicrobial activities under the
prevailing conditions of the test.
(2) Carry
out the filtration as rapidly as possible as per details given under ‘Oils and Oily Solu-tions’ [Section
III] from step (4) onwards.
(3) However,
in certain exceptional instances, it would be absolutely necessary to heat the
substance to not more than 45°C, and
to make use of ‘warm solutions’ for
washing the membrane effectively.
Note : For ointments and oils that are almost
insoluble in isopropyl myristate one may employ the second method viz., ‘Direct Inoculation’ [Section 2.2].
[V] Soluble Soids : For each
individual cultrue medium, dissolve not less the quantity of the substance being examined, as
recommended in Tables : 8.2 and 8.3, in an appropriate sterile solvent e.g.,
fluid A, and perform the test described under Section (I) i.e., Aqueous Solutions, by employing a membrane suitable for
the selected solvents.
[VI] Sterile Devices : Pass
carefully and aseptically a sufficient volume of fluid B via each of not less than 20 devices so that not less than 100 mL is recovered
ultimately from each device. Collect the fluids in sterile containers, and
filter the entire volume collected via
membrane filter funnel(s) as
described under Section (I), Aqueous Solutions.
2. Direct Inoculation [or Direct Inoculation of Culture Media]
The three usual methods being used for
performing the ‘tests for sterility’
are as enumerated under :
(a) Nutrient
Broth,
(b) Cooked
Meat Medium and Thioglycollate Medium, and
(c) Sabouraud Medium.
These
methods shall now be treated individually in the sections that follows :
2.1. Nutrient Broth
Importantly,
it is exclusively suitable for the ‘aerobic
microorganisms’.
·
Oxidation-reduction potential (Eh) value
of this medium happens to be quite high to enable the growth of the anaerobes specifically.
·
Importantly, such culture media that particularly
allow the growth of festidious
microor-ganisms, such as : soyabean
casein digest broth, Hartley’s digest broth.*
2.2. Cooked Meat Medium and Thioglycollate Medium
These two different types of media are discussed
briefly as under :
(a) Cooked Meat Medium : It is
specifically suited for the cultivation (growth) of clostridia**.
(b) Thioglycollate Medium : It is
particularly suited for the growth of anaerobic
microbes. It essentially comprises of the following ingredients, namely :
Glucose and Sodium thioglycollate— that
invariably serve as :
·
an inactivator
of mercury compounds,
·
to augment and promote
reducing parameters, and
·
an oxidation-reduction
indicator.
Agar—to cause reduction of the
ensuing ‘convection currents’.
2.3. Sabouraud Medium
It is a
medium specifically meant for fungal
species. It essentially bears two
vital and important characteristic features, such as :
·
an acidic
medium, and
·
contains a rapidly
fermentable carbohydrate e.g.,
glucose or maltose.
Note : (1) All the three aforesaid media must be
previously assessed adequately for their nutritive characteristic features i.e., in fertility tests to ascertain
the growth of specified microorganisms.
(2) Duly incubated at the stipulated temperature(s).
The direct inoculation method shall now be
dealt with in a sequential manner under the following three categories, such as :
·
Quantities of sample to be employed,
·
Method of test, and
·
Observation and Interpretation of Results.
Quantities of Sample to be used : In actual
practice, the precise quantum of the
substance or pharmaceutical
preparation under investigation, that is required to be used for inoculation in the respective culture
media usually varies justifiably as per the amount present in each
particular con-tainer, and is stated clearly in Table : 8.2 together with the
exact volume of the culture medium to be employed.
Method of Test : The ‘method of test’ varies according to the substance to be examined,
for instance :
(a) Aqueous Solutions and Suspensions : The
actual tests for microbial contamination
are invariably performed on the
same sample of the preparation under investigation by making use of the
above-stated media (Section 2.2.1 through 2.2.3). In certain specific instance
when the amount present in a single container is quite insufficient to carry
out the stipulated ‘tests’, the
combined contents of either two or mroe containers may be employed to inoculate
the above-stated media.
Methodology : The various sequential steps involved
are as given under :
(1) Liquid
from the ‘test containers’ must be
removed carefully with a sterile pipette or with a sterile syringe or a needle.
(2) Transfer
aseptically the requistite prescribed volume of the substance from each
container to a vessel of the culture medium.
(3) Mix
the liquid with the medium carefully taking care not to aerate excessively.
(4) Incubate
the ‘inoculated media’ for not less
than 14 days (unless otherwise specifically mentioned in the monograph*) at : 30–35°C for ‘Fluid Thioglycollate Medium’, and
20–25°C for ‘Soyabean-Casein Digest
Medium’.
Special Points : The following special points may be noted meticulously :
(i) In
case, the substance under investigation renders the culture medium turbid whereby the presence or absence
of the actual microbial growth may
not be determined conveniently and readily by sheer ‘visual examination’, it is always advisable and recommended that a
suitable transfer of a certain portion of the medium to other fresh vessels of
the same medium between the 3rd and
7th days after the said test actually commenced.
(ii) Subsequently,
continue the incubation of the said ‘transfer
vessels’ for not less than 7
addi-tional days after the transfer, and for a total of not less than 14 days.
(b) Oils and Oily Solutions : For
carrying out the required tests for the bacterial contamination of oils and oily solutions it is recommended to make use of culture media to which have been
incorpo-rated duly :
Octylphenoxy
polyethoxyethanol (I) : 0.1% (w/v) [or Octoxynol]
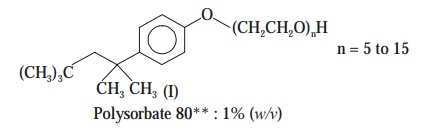
However,
these emulsifying agents should not exhibit any inherent antimicrobial
characteristic features under the prevailing parameters of the ‘test’.
The
required test must be carried out as already described under Section (a) above i.e., Aqueous Solutions and Suspensions.
Precautionary Measures : The
following two precautionary measures should
be taken adequately :
(i) Cultures essentially comprising of ‘oily preparations’ should be shaken
gently every day.
(ii) Importantly,
when one employs the fluid
thioglycollate medium for the ultimate detection of the anaerobic microorganisms, shaking or
mixing must be restricted to a bear minimum level so as to maintain perfect anaerobic experimental parameters.
(c) Ointments : The following steps may be
adopted in a sequential manner :
(1) Carefully
prepare the ‘test sample’ by
diluting ten times in a sterile diluent, for instance : Fluid B or any other suitable aqueous vehicle which is capable of
dispersing the test material homogene-ously throughout the ‘fluid mixture’.*
(2) Mix
10 mL of the fluid mixture thus
obtained with 80 mL of the medium, and subsequently proceed as per the method
given under Section (a) i.e., Aqueous Solutions and Suspensions.
(d) Solids : The various steps involved are as
stated under :
(1) Transfer
carefully the requisite amount of the preparation under examination to the
quantity of culture medium as specified in Table : 8.3, and mix thoroughly.
(2) Incubate
the inoculated media for not less than 14 days, unless otherwise mentioned in
the monograph at 30–35°C in the particular instance of fluid thioglycollate medium, and at 20–25°C in the specific case of
soyabean-casein digest medium.
(e) Sterile Devices : For
articles of such size and shape as allow the complete immersion
in not more than 1 L of the culture
medium test the intact article, using the suitable media ; and incubating as
stated under Section (a) i.e., Aqueous Solutions and Suspensions.
(f) Transfusion or Infusion Assemblies : For transfusion or infusion assemblies or
where the size of an item almost
renders immersion impracticable, and exclusively the ‘liquid pathway’ should be sterile by all means, flush carefully
the lumen of each of twenty units
with a sufficient quantum of fluid thioglycollate medium and the lumen of
each of 20 units with a sufficient
quantum of soyabean-casein digest medium
to give an ultimate recovery of not less than 15 mL of each medium.
Finally, incubate with not less than
100 mL of each of the two media as prescribed under Section (a) i.e.,
Aque-ous Solutions and Suspensions.
Exception : Such ‘medical devices’ wherein the lumen is so small such that fluid thioglycollate medium will not
pass through easily, appropriately substitute alternative thioglycollate medium instead of the usual fluid thioglycollate medium and incubate
that duly inoculated medium
anaerobically.
Note : In such situations where the presence of the
specimen under examination, in the culture medium critically interferes with
the test by virtue of the ensuing bacteriostatic or fungistatic action, rinse
the article thoroughly with the bare minimum quantum of fluid A. Finally recover the rinsed fluid and carry out the ‘test’
as stated under ‘Membrane Filtration’ for Sterile Devices.
Observation and Interpretation of Results : In the
case of ‘direct inoculation’ the
various observation and interpretation
of results may be accomplished by taking into consideration the fol-lowing cardinal factors, such as :
(1) Both
at intervals during the incubation
period, and at its completion,
the media may be examined thoroughly for the critical macroscopic evidence of the bacterial growth.
(2) In
the event of a negative evidence,
the ‘sample’ under examination
passes the ‘tests for sterility’.
(3) If positive
evidence of microbial growth is found, reserve the containers exhibiting this,
and unless it is amply proved and adequately demonstrated by any other means
that their (microorganisms) presence is on account such causes unrelated to the
‘sample’ being examined ; and,
therefore, the tests for sterility are pronounced invalid. In such cases, it may be
recommended to carry out a ‘retest’ employing
an identical number of samples and
volumes to be tested, and the media as in the original test.
(4) Even then,
if no evidence of microbial growth
is duly observed, the ‘sample’ under
inves-tigation precisely passes the ‘tests for sterility’.
(5) In
case, reasonable evidence of bacterial growth is observed, one may go ahead
with the isolation and subsequent identification of the organisms.
(6) If
they are found to be not readily distinguishable from those (microbes) growing
in the containers reserved in the very First
Test, the ‘sample’ under
investigation fails the ‘tests for sterility’.
(7) In
case, the microorganisms are readily distinguishable from the ones actually
growing in the containers reserved in the ‘First
Test’, it is very much advisable to carry out a ‘Second Retest’ by employing virtually twice the number of samples.
(8) Importantly,
if no evidence of bacterial growth
is observed in the ‘Second Retest’,
the sample under examination legitimately passes
the ‘tests for sterility’.
(9) Contrarily,
if evidence of growth of any
microorganisms is duly observed in the ‘second
retest’, the sample under investigation obviously fails the ‘tests for sterility’.
Related Topics
