Peptidoglycan biosynthesis in bacteria and its inhibition
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Mechanisms of action of antibiotics and synthetic anti-infective agents
Peptidoglycan is a vital component of the cell wall of virtually all bacteria. About 50% of the weight of the wall of Gram-positive bacteria is peptidoglycan; smaller amounts occur in mycobacterial walls (30%) and Gram-negative bacterial cell walls (10-20%).
PEPTIDOGLYCAN BIOSYNTHESIS IN BACTERIA AND ITS INHIBITION
Peptidoglycan is a vital component of
the cell wall of virtually all bacteria. About 50% of the weight of the wall of
Gram-positive bacteria is peptidoglycan; smaller amounts occur in mycobacterial
walls (30%) and Gram-negative bacterial cell walls (10-20%). It is a
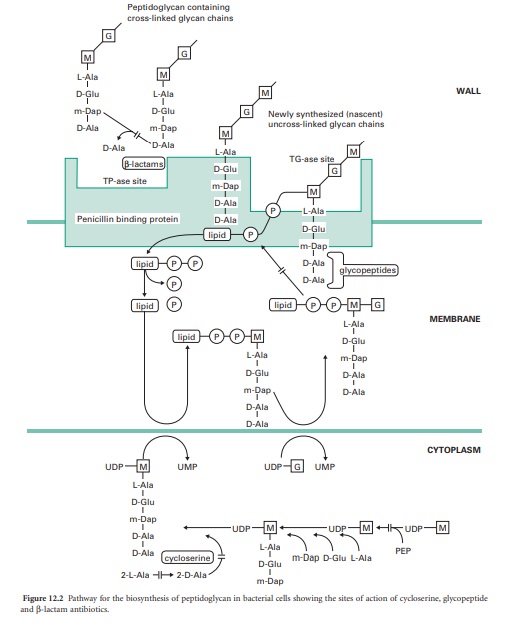
macromol-Figure 12.1 Schematic diagram of a fusidic acid short peptide chains (Figure 12.2).
The glycan chains contain alternating units of N-acetylmuramic acid and
N-acetylglucosamine. Each N-acetylmuramic acid contains a short peptide
substituent made up of four amino acids (the stem peptides). A key feature of
peptidoglycan is the occurrence of the d-isomers of some amino acids in the
stem peptides (particularly d-alanine and d-glutamic acid) and unusual amino
acids such as meso-diaminopimelic acid which are not found in proteins. In some
organisms (e.g. Escherichia coli) cross-linking of the stem peptides involves a
direct peptide bond between the fourth amino acid of the stem peptide on one
chain and the third amino acid in the stem peptide on an adjacent chain. In
other organisms (e.g. Staphylococcus aureus) the linkage is made by a short
peptide bridge (e.g. five glycines) between the stem peptides. The precise
composition of peptidoglycan varies between different organisms but the overall
structure is the same.
In all cases the peptidoglycan plays a vital role: it is responsible for
maintaining the shape and mechanical strength of the bacterial cell. If it is
damaged in any way, and particularly if its synthesis is inhibited, then the
shape of the cells becomes distorted, they swell and will eventually burst
(lyse) as a result of the high internal osmotic pressure. Mammalian cells do
not possess a cell wall and contain no other macromolecules resembling
peptidoglycan. Consequently, antibiotics which interfere with the synthesis and
assembly of peptidoglycan show excellent selective toxicity.
D-Cycloserine
D-Cycloserine interferes with the early stage of synthesis of
peptidoglycan involving the assembly of the dipeptide d-alanyl-D-alanine. This
occurs inside the cytoplasm and involves a racemase enzyme which converts
l-alanine to d-alanine and a ligase which couples two d-alanines together
(Figure 12.2). Both of these enzymes are inhibited by d-cycloserine, which
bears some structural similarities to D-alanine. The antibiotic binds to the
pyridoxal phosphate cofactor of the enzymes, effectively preventing them from
forming d-alanyl-D-alanine. Subsequent stages of peptidoglycan synthesis,
involving coupling of the dipeptide to three other amino acids forming the stem
peptide on UDP-N-acetylmuramicacid,areblocked. Note that initially the peptide
contains five amino acids, terminating in d-alanyl-d-alanine.Theterminal
D-alanine is removed on insertion into the cell wall during the final step in
which the cross-links are formed between stem peptides on adjacent glycan
strands.
Glycopeptides—vancomycin and teicoplanin
The peptidoglycan macromolecule is assembled in the cell wall by the
sequential action of two enzymes (transglycosylases and transpeptidases) which
are located on the outer face of the cytoplasmic membrane. To reach the assembly
site (i.e. a region of cell wall growth) the precursors, which are assembled in
the cytoplasm, must cross the cell membrane. They do this linked to a lipid,
undeca-prenylphosphate, which acts as a carrier molecule, cycling between the
inner and outer faces of the membrane. The biochemical details of this process
are outlined in Figure 12.2. Antibiotics interfering with this stage of
peptidoglycan synthesis have been identified, e.g. bacitracin, but they have
not found major applications in the treatment of infections.
The glycopeptides vancomycin and teicoplanin act at the stage where the
peptidoglycan precursors are inserted into the cell wall by the trans-glycosylase
enzyme on the outer face of the cell membrane. This enzyme assembles linear
glycan chains that are not initially cross-linked to the existing peptidoglycan
in the cell wall. The linear glycan chains are assembled by the trans-glycosylase
by transfer of the growing glycan chain to the disaccharide peptidoglycan
precursor on the lipid carrier as it crosses the cell membrane. Glycopeptides
block this process by binding, not to the enzyme itself, but to the
disaccharide peptidoglycan precursor, specifically to the d-alanyl-d-alanine
portion on the stem peptide. The presence of the bulky glycopeptides tightly
bound to each d-alanyl-d-alanine residue prevents the trans-glycosylase from
carrying out the transfer reaction. Binding involves formation of a network of
five hydrogen bonds between amino acid residues on the glycopeptide antibiotics
and d-alanyl-d-alanine. Resistance to this unusual mecha-nism of enzyme
inhibition can result from alteration in the d-alanyl-d-alanine substrate to
d-alanyl-d-lactate, which occurs in glycopeptide (e.g. vancomycin)-resistant
enterococci (VRE). Vancomycin does not penetrate the cell membrane of bacteria
and is thought to bind to the disaccharide-pentapeptides on the outer face of
the cytoplasmic membrane. It has been suggested that two vancomycin molecules
form a back-to-back dimer which bridges between pentapeptides on separate
glycan chains, thus preventing further peptidoglycan assembly. Teicoplanin also
binds tightly to the d-alanyl-d-alanine region of the peptidoglycan precursor.
However, as a lipoglycopeptide it may act slightly differently from vancomycin,
by locating itself in the outer face of the cytoplasmic membrane and binding
the pentapeptide as the precursors are transferred through the membrane.
Glycopeptides must cross the cell wall to reach the outer face of the cell
membrane where trans-glycosylation takes place. They are too large to penetrate
the outer membrane of most Gram-negative bacteria and are consequently used for
treatment of serious Gram-positive infections.
β-Lactams—penicillins, cephalosporins, carbapenems and monobactams
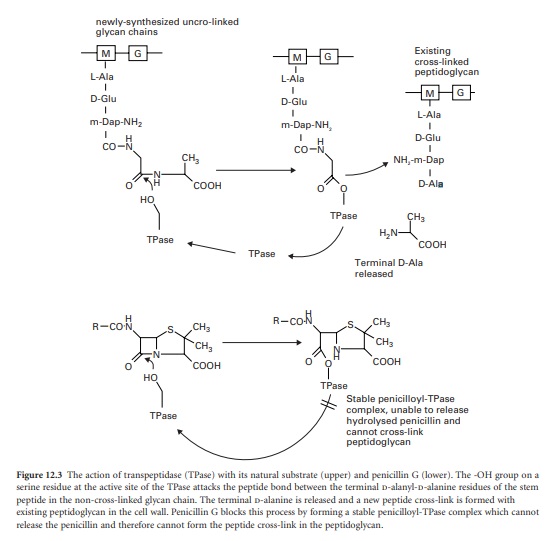
The final stage of peptidoglycan assembly is the cross-linking of the
linear glycan strands assembled by trans-glycosylation to the existing
peptidoglycan in the cell wall. This reaction is catalysed by transpeptidase
enzymes, which are also located on the outer face of the cell membrane. They
first remove the terminal d-alanine residue from each stem peptide on the newly
synthesized glycan chain. The energy released from breaking the peptide bond
between the two alanines is used in the formation of a new peptide bond between
the remaining D-alanine on the stem peptide and a free amino group present on
the third amino acid of the stem peptides in the existing cross-linked
peptidoglycan. In many organisms, including E. coli, this acceptor amino group
is supplied by the amino acid diaminopimelic acid. In other organisms, e.g.
Staph. aureus, the acceptor amino group is supplied by the amino acid l-lysine.
Although there is considerable variation in the composition of the peptide
cross-link among different species of bacteria, the essential transpeptidation
mechanism is the same. Therefore, virtually all bacteria can be inhibited by
interference with this group of enzymes.
The β-lactam antibiotics inhibit
transpeptidases by acting as alternative substrates. They mimic the
d-alanyl-d-alanine residues and react covalently with the transpeptidases (Figure 12.3).
The β-lactam bond (common to all members of the β-lactam antibiotics) is broken
but the remaining portion of the antibiotic is not released immediately. The
half-life for the transpeptidase-antibiotic complex is of the order of 10
minutes; during this time the enzyme cannot participate in further rounds of
peptidoglycan assembly by reaction with its true sub-strate. The vital
cross-linking of the peptidoglycan is therefore blocked while other aspects of
cell growth continue. The cells become deformed in shape and eventually burst
through the combined action of a weakened cell wall, high internal osmotic
pressure and the uncontrolled activity of autolytic enzymes in the cell wall.
Penicillins, cephalosporins, carbapenems and monobactams all inhibit
peptidoglycan cross-linking through interaction of the common β-lactam ring
with the transpeptidase enzymes. However, there is considerable variation in
the morphological effects of different β-lactams owing to the existence of
several types of transpeptidase. The transpeptidase enzymes are usually
referred to as penicillin binding proteins (PBPs) because they can be separated
and studied after reaction with 14C-labelled penicillin. This step is necessary
because there are very few copies of each enzyme present in a cell. They are
usually separated according to their size by electrophoresis and are numbered
PBP1, PBP2, etc., starting from the highest molecular weight species. In
Gram-negative bacteria most of the high molecular weight transpeptidases also
possess trans-glycosylase activity, i.e. they have a dual function in the final
stages of peptidoglycan synthesis with the trans-glycosylase and transpeptidase
activities located in separate regions of the protein structures. Furthermore,
the different transpeptidases have specialized functions in the cell; all
cross-link peptidoglycan but some are involved with maintenance of cell
integrity, some regulate cell shape and others produce new cross wall between
elongating cells, securing chromosome segregation prior to cell division. The
varying sensitivity of the PBPs towards different β-lactams helps to explain
the range of morphological effects observed in treated bacteria. For example,
penicillin G (benzylpenicillin), ampicillin and cephaloridine are particularly
effective in causing rapid lysis of Gram-negative bacteria such as E. coli.
These antibiotics act primarily upon PBP1B, the major transpeptidase of the
organism. Other β-lactams have little activity against this PBP, e.g.
mecillinam binds preferentially to PBP2 and it produces a pronounced change in
the cells from a rod shape to an oval form. Many of the cephalosporins, e.g.
cephalexin, cefotaxime and ceftazidime, bind to PBP3 resulting in the formation
of elongated, filamentous cells. The lower molecular weight PBPs, 4, 5 and 6,
do not possess transpeptidase activity. These are carboxypeptidases, which
remove the terminal D-alanine from the pentapeptides on the linear glycans in
the cell wall but do not catalyse the cross-linkage. Their role in the cells is
to regulate the degree of cross-linking by denying the d-alanyl-d-alanine
substrate to the transpeptidases but they are not essential for cell growth. Up
to 90% of the amount of antibiotic reacting with the cells may be consumed in
inhibiting the carboxypeptidases, with no lethal consequences to the cells.
Gram-positive bacteria also have multiple transpeptidases, but fewer
than Gram-negatives. Shape changes are less evident than with Gram-negative
rod-shaped organisms. Cell death follows lysis of the cells mediated by the
action of endogenous autolytic enzymes (autolysins) present in the cell wall
which are activated following β-lactam action. Autolytic enzymes able to hydrolyse
peptidoglycan are present in most bacterial walls; they are needed to re-shape
the wall during growth and to aid cell separation during division. Their
activity is regulated by binding to wall components such as the wall and
membrane teichoic acids. When peptidoglycan assembly is disrupted through
β-lactam action, some of the teichoic acids are released from the cells, which
are then susceptible to attack by their own autolysins.
β-Lactamase inhibitors—clavulanic acid, sulbactam and tazobactam
Expression of β-lactamase enzymes is the most important mechanism
through which organisms become resistant to β-lactams. Over 300 different β-lactamase
enzymes have been described and they can be classified either by amino acid
sequence or by their biochemical properties. The majority of the enzymes have a
serine residue at their active site and bear structural and mechanistic
similarities to the carboxypeptidases from which they are thought to have
evolved. Unlike the transpeptidases and carboxypeptidases, the β-lactamases
hydrolyse β-lactam antibiotics very efficiently, releasing fragments of the
antibiotics rapidly instead of remaining bound to the ring opened forms for
several minutes. A number of successful inhibitors, including clavulanic acid,
sulbactam and tazobactam have been developed for use in combination with
susceptible β-lactams (amoxicillin, ampicillin and piperacillin, respectively),
protecting them from inactivation by the β-lactamases. The inhibitors are
hydrolysed by the β-lactamases in the same manner as susceptible β-lactam
antibiotics, the β-lactam ring being broken by attack by a serine residue in
the active site of the enzyme. Instead of undergoing rapid release from the
active site serine, the inhibitors remain bound and undergo one of several
different fates. It is thought that the hydrolysed inhibitors can interact with
a second enzyme residue in the active site of the β-lactamase, forming a
covalently cross-linked, irreversibly inhibited complex. Other categories of
β-lactamase enzymes have zinc atoms at their active sites and hydrolyse the
β-lactam ring by a different mechanism to the serine based enzymes. These
metallo-β-lactamases are not inhibited by clavulanic acid, sulbactam and
tazobactam.
Related Topics
