Physicochemical properties and solubility
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Pharmaceutical polymers
The chemical reactivity of polymers depends on the chemistry of their monomer units.
Physicochemical
properties and solubility
The
chemical reactivity of polymers depends on the chemistry of their monomer
units. However, their physicochemical properties depend, to a large extent, on
the way the monomeric units are put together. This con-tributes to the
versatility of synthetic polymers. Various arrangements of monomers, shown as ○
and ● in Figure 11.2, can be produced with
consequent effects on the physical properties of the resulting polymer. For
example, Eudragit polymers are commercially available with a wide range of
physicochemical properties, including the effect of pH on solubility.
Depending
on the polymer type and the types of cosolvents used, the over-all solubility
of a single polymer or a mixture of polymers can be altered in a formulation.
Polymers that are sufficiently polar interact with the water through hydrogen
bonding to provide the favorable negative energy of sol-vation; that is, energy
is released on dissolution of such polymers in water. Water-soluble polymers
tend to increase the viscosity of their solutions as a function of polymer
concentration due to entanglement and bonding of solvent molecules and
reduction of slip planes in the colloidal polymer solu-tions. Hydrophilic
polymers also tend to swell by absorbing water in solu-tion. In contrast,
water-insoluble or hydrophobic polymers tend to form thin films or matrices.
Figure 11.4 represents polymer morphologies in solution,
gel, and solid states. In solution, the polymer conformation depends on the
interaction between the polymer and the solvent and whether the polymer chains
asso-ciate to form micelles. Covalent cross-linking, hydrogen bonding,
entangle-ment, or hydrophobic interactions among polymer chains in solution
form
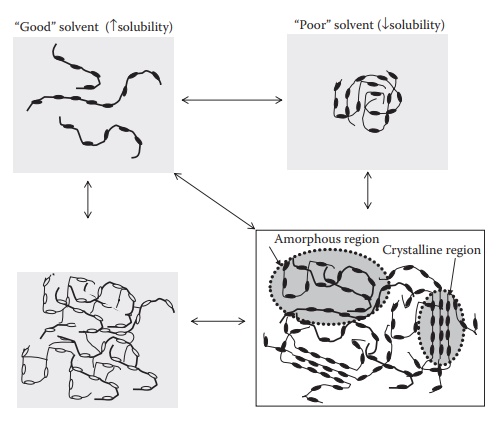
Figure 11.4 Representation of polymer morphologies in solution, gel (hydrogel or lipogel), and solid states. In solution, the polymer conformation depends on the polymer-solvent interaction and whether the polymer chains associate to form micelles. Gels can be formed by covalent cross-linking, hydrogen bonding, or hydrophobic interactions.
Related Topics
