Intelligent or stimuli-sensitive polymers
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Pharmaceutical polymers
The terms intelligent, stimuli-sensitive, or stimuli-responsive polymers refer to polymers that exhibit relatively large and sharp changes in physical or chemical properties in response to a small change in the environment, such as pH and temperature.
Intelligent or
stimuli-sensitive polymers
The
terms intelligent, stimuli-sensitive, or stimuli-responsive polymers refer to
polymers that exhibit relatively large and sharp changes in physical or
chemical properties in response to a small change in the environment, such as
pH and temperature. Changes in the environment that affect polymer properties
are termed stimuli, while the resulting changes in the polymer and the system
(such as dissolved state of the polymer in a sol-vent) are termed the
responses. The mechanistic basis of changes in the physical properties of
stimuli-responsive polymers is generally a modifi-cation in the structure of
the polymer in solution. For example, water-soluble polymers and copolymers can
undergo conformational change or phase transition in response to environmental
stimuli. These changes may exhibit as swelling, change in solubility and
conformation of poly-mer matrix or chain, or polymer precipitation. When a
soluble polymer is stimulated to precipitate, it will be selectively removed
from the solution. When such polymers are grafted or coated onto a solid
support, then one may reversibly change the water adsorption into the
polymer-coated surface of the solid, thus changing the wettability of the
surface. When a
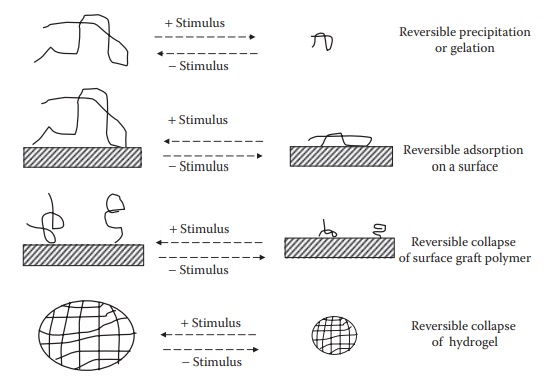
Figure 11.6 Schematic representations of stimuli-sensitive polymers in solutions,
on surface, and as hydrogels.
hydrogel
is stimulated to collapse, it shrinks in size, squeezes out water from its
pores, turns opaque, and becomes stiffer. Figure 11.6
shows the schematic representations of stimuli-sensitive polymers in solutions,
on surface, and as hydrogels.
The
stimuli responsiveness of the stimuli-sensitive polymers originates in the bulk
and surface chemistry and the architecture of these organic compounds.
Physicochemical properties of organic compounds are a result of their surface
chemistry (i.e., elemental and functional group compo-sition) and architecture
(i.e., surface exposure of functional groups and molecular domains). The
surface exposure or display of functional groups could be different within the
different forms of the same molecule. For small molecules, this is exemplified by
the existence of different crystalline forms (polymorphism) and different
morphologies of the same crystalline form. These result in different surface
properties (such as solubility and dissolution rate) and bulk properties (such
as powder adhesion) of crys-tals, depending on the differences in the
functional groups and molecular domains exposed on the surface.1 For large molecules, this is exemplified by protein
structure where surface exposure of functional groups of a peptide chain can
lead to a protein being hydrophilic and globular or hydropho-bic and
membrane-embedded, with many potential secondary and tertiary structure
possibilities.
In
addition to the changes in these physicochemical properties, for rela-tively
large-molecular-weight organic compounds (such as polymers and proteins), the
sheer multitude of functional groups and presence of molecu-lar domains (such
as hydrophobic regions) impart special characteristics to the interactions of
these molecules with the external environment. These interactions include
solute–solute and solute–solvent interactions in the dissolved state. Such
interactions are responsible for phenomena such as micellization and
swelling/collapse of a cross-linked scaffold.
At
a molecular level, stimuli responsiveness of polymers is typically based on
changes in polymer–polymer and polymer–solvent interac-tions. For example,
polymers that bear multiple ionizable weakly acidic or weakly basic functional
groups undergo ionization as a function of pH, resulting in changes in the
strength and extent of polymer interactions with the solvent. Polymer
structure, number and positioning of the func-tional groups, and the strength
of their interactions determine the macro-scopic response of the polymer system
to the environmental stimulus. For example, some polymeric systems can undergo
reversible or irreversible phase transformation from a single-phase solution to
biphasic precipitated or aggregated state. However, certain polymer solutions
may display gell-ing or micellization without physical phase separation. In
addition, colloi-dal or biphasic polymeric systems that contain cross-linked
polymers can show polymer swelling or shrinkage with changes in the type and
strength of polymer–solvent interactions.
pH-responsive polymers
Polymers
that bear ionizable functional groups can undergo reversible (e.g., by
hydration) or irreversible (e.g., by hydrolysis) changes in response to changes
in the environmental pH. Hydration can lead to reversible poly-mer swelling and
deswelling or collapse. Hydrolysis, on the other hand, can lead to irreversible
polymer-chain degradation or breakage, result-ing in changes in the overall
molecular weight and monomer content of the polymer. The reversible
pH-sensitive polymers are typically polyelec-trolytes that contain a multiple
weakly acidic or weakly basic (ionizable) functional groups, whose ionization
status can change in response to the environmental pH. The weak acid, HA, is
ionized at basic pH to H+ and A−, while the weak base, B,
is ionized at acidic pH to BH+. In the ionized state, increased
polymer–water interaction through hydrogen and electro-static bond interactions
leads to higher proportion of polymer-associated water of hydration. In
addition, electrostatic repulsion between functional groups bearing the same
charge on the polymer backbone can lead to polymer-chain expansion. In the
unionized state, weak dipole–dipole and hydrophobic interactions within and
between the polymer chains can lead to polymer collapse, solvent exclusion, and
reduced hydrodynamic volume, eventually causing polymer aggregation or
precipitation.
Changes
in polymer properties, such as water solubility, water absorp-tion, and polymer
degradation by hydrolysis, as a function of solution pH can be utilized in
various ways. For example:
·
Sustained drug
delivery can
be achieved by forming a well-mixed matrix
or a core–shell structure of a water-soluble drug in a water-insoluble polymer
that degrades in the appropriate pH environment. Hydrolytic degradation of the
polymer leads to slow and sustained drug release.
·
pH-triggered drug
release in
the target tissue can be achieved using polymers
linked to drugs through hydrolyzable functional groups. For example, polymeric
prodrugs of 5-amino salicylic acid (5-ASA) such as methacryloyloxyethyl-5-amino
salicylate (MOES) and N-methacryloylaminoethyl-5-amino
salicylamide (MAES) utilize hydrolyzable
ester linkages that release the drug in the colon on cleav-age of the
biodegradable linkers.
·
Tumor tissue
targeting of
a cytotoxic drug can be achieved by cova-lent conjugation with or entrapment in
the drug delivery system com-posed of a pH-sensitive polymer. Tumor tissues
have a slightly lower pH compared to nontumor tissues. The polymers that show
signifi-cant change in their physicochemical properties as a result of decrease
in the environmental pH, usually due to the ionization of basic func-tional
groups, can be utilized for targeted drug delivery to the tumors.
·
Intracellular drug
release in
specific organelles or regions of the cell
can be achieved based on the mechanism of cellular uptake and the
pH-responsive properties of the polymer utilized in a drug deliv-ery system.
The pH responsiveness of polymers has been utilized in nonviral gene and
antisense drug delivery by utilizing polycationic polymers, such as
poly(ethyleneimine) (PEI), poly(l-lysine) (PLL), and poly(l-histidine) (PLH).
These polymers possess multiple amine func-tional groups that are cationically
(positively) charged and ionized at acidic pH but unionized at basic and neutral
pH. On cellular uptake through the endosomal pathway, as the pH of the
endosomes becomes more and more acidic toward lysosomal pH, the ionization of
these polymers leads to water retention and increase in osmotic pressure of the
endosomal vesicles, causing their disruption. These carriers, then, are able to
release the drug cargo intracellularly before the endosomes become the
lysosomes.
·
Enteric- and
colon-targeted oral drug delivery can be achieved by coating a drug delivery system using a
polymer that exhibits pH-dependent solubility or degradation. Gastrointestinal
(GI) fluid’s pH changes progressively from acidic to basic from the stomach
through the intestines to the colon. The changes in the GI fluid’s pH can be
utilized to release a drug at a particular physiological location in the GI
tract. In particular, dosage forms of drugs that are sensitive to the acidic
environment of the stomach, or whose release in the stom-ach is otherwise
undesirable, can be coated with a polymer that would be insoluble at the acidic
stomach pH and soluble at the basic intes-tinal pH. Such polymers are known as
enteric polymers, and such a coating on the dosage form is termed enteric
coating. Enteric poly-mers are exemplified by methacrylic acid polymer
(Eudragit® L100), methyl methacrylate polymer (Eudragit®
S100), CAP, HPMCP, and carboxymethyl ethylcellulose etc.
Thermosensitive polymers
Thermosensitive
polymers show changes in physicochemical properties with pharmaceutically and
physiologically relevant changes in tempera-ture. An aqueous polymer solution
might show phase transition above or below a certain temperature, called the critical solution temperature (CST).
Solutions may exhibit upper or lower CST, depending on their temperature.
Solutions that exhibit upper CST (UCST) are monophasic and isotropic above a
certain temperature (but biphasic below that temperature), while solutions that
exhibit a lower CST (LCST) are monophasic below a certain temperature (and
biphasic above that temperature). The CST is also known as the cloud point, since phase separation
occurs at this temperature, lead-ing to cloudy appearance of the solution.
Injectable
depot formulations, tissue engineering, and temperature-induced tumor drug
delivery exemplify biopharmaceutical applications of thermoresponsive polymers.
Temperature-responsive biodegradable poly-mers that show sol–gel transition
between room temperature and physi-ological temperature can be utilized to
prepare a drug formulation as a solution that precipitates or forms a gel on
injection. The in vivo gel serves as
a drug reservoir for sustained or controlled drug release over a period of
time. Injectable gel-forming polymers are exemplified by block copo-lymers of
poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO). Commercially
available injectable polymers that undergo sol–gel transition include BST-Gel®
(BioSyntech) and ReGel® (Macromed) etc.
Other stimuli-responsive polymers
Light-responsive
polymers can be designed to respond to either visible or ultraviolet (UV) light.
This behavior is typically exhibited in a polymer solution or hydrogel.
Hydrophilic polymers can form hydrogels in water, which are three-dimensional
polymer chain networks with unique physical properties such as higher viscosity
and reduced fluidity than liquid water or dilute polymer solutions. The
mechanism of stimuli responsiveness of light-responsive hydrogels may involve
chemical bond cleavage with the higher-energy UV irradiation or may involve
energy transfer to the polymer network with lower-energy visible radiation.
Electrically
responsive polymers respond to the application of electrical field for
triggering or controlling drug release. These allow user control over the
amplitude of current, duration of impulses, and the time interval between
electrical impulses. Drug delivery systems that respond to elec-trical signal
are typically polyelectrolyte polymers with multiple ionizable functional
groups. The application of electric field aligns the dipoles in polar or
charged molecules, resulting in a change in the polymer conforma-tion. This
conformational change could involve polymer swelling, shrink-age, or a change
in shape. A small change in the electric potential across a polyelectrolyte gel
can lead to significant (up to several 100-fold) reversible change in the
volume of the gel.
Magnetically
responsive drug delivery systems have been used to target the location of drug
release to a particular tissue and/or trigger the drug release from a system
based on the application of an exter-nal magnetic field. This is usually
accomplished by the incorporation of ferromagnetic micro- or nanoparticles in
the drug delivery system. Magnetically responsive drug delivery systems might
require exposure to strong magnetic fields for prolonged period of time. This
exposure is likely to generate localized heating, which could be a part of the
drug-release mechanism. For example, Katagiri et al. showed drug release from
magnetically responsive lipid bilayer capsules prepared with mag-netite, iron
(II, III) oxide (Fe3O4), nanoparticles. The lipid bilayer
was deposited on top of the magnetite nanoparticles. On application of
alter-nating magnetic field, heating of the magnetite resulted in phase
transi-tion of the bilayer membrane, leading to release of the drug incorporated
in the bilayers.
Ultrasound-responsive
drug delivery systems commonly use ultrasound as a permeation enhancer through
biological membranes such as skin, lungs, intestinal wall, and blood vessels.
The use of ultrasound for increas-ing transdermal drug delivery is known as sonophoresis or phonophore-sis. Ultrasound increases skin permeability through
formation of bubbles caused by
acoustic cavitation. The use of ultrasound for drug delivery can be based on
energy transfer from the ultrasonic waves, leading to chemical degradation of
the polymer.
Related Topics
