Pool Boiling
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Heat Transfer
If a horizontal heating surface is in contact with a boiling liquid, a sequence of events occurs as the temperature difference between the surface and the liquid increases.
POOL BOILING
If a horizontal
heating surface is in contact with a boiling liquid, a sequence of events
occurs as the temperature difference between the surface and the liquid
increases. Figure 3.3 relates heat flux per unit area at the surface, q, to the temperature difference between
the surface and boiling water, ΔT.
The derived value of the heat transfer coefficient, h = q/ΔT, is also plotted.
When
ΔT is small, the
degree of superheating of the liquid layers adjacent to the surface is low, and
bubble formation, growth, and disengagement, if present, are slow. Liquid
disturbance is small, and heat transfer can be estimated from expressions for
natural convection given, for example, in equation (3.11). This regime
corresponds to section AB of Figure 3.3, over which both q and h increase.
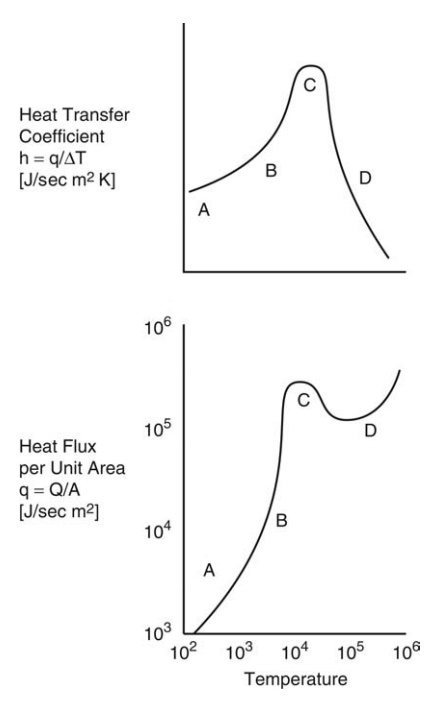
FIGURE 3.3 Variation in heat transfer coefficient and heat flux per unit area.
In
section BC of Figure 3.3, vapor formation becomes more vigorous and bubble
chains rise from points that progressively increase in number and finally
merge. This movement increases liquid circulation, and both q and h rise
rapidly. This phase is called nucleate boiling and is the practically important
regime. For water, approximate values of q
and h may be read from Figure 3.3. At
point C, a peak flux occurs and a maximum heat transfer coefficient is
obtained. ΔT at this point is
known as the critical temperature drop. For water, the value lies between 25
and 32 K. The critical temperature drop for organic liquids is somewhat higher.
Beyond C, vapor formation is so rapid that escape is inadequate and a progressively
larger fraction of the heating surface becomes covered with a vapor film, the
low conductivity of which leads to a decrease in q and h. This represents a
transition from nucleate boiling to film boiling. When this transition is
complete (D), the vapor entirely covers the surface, film boiling is fully
established, and the heat flux again rises.
The
low heat transfer coefficient renders film boiling undesirable, and equipment
is designed for and operated at temperature differences that are less than the
critical temperature drop. If a constant temperature heat source, such as steam
or hot liquid, is employed, exceeding the critical temperature drop results
simply in a drop in heat flux and process efficiency. If, however, a constant
heat input source is used, as in electrical heating, decreasing heat flux as
the tran-sition region is entered causes a sudden and possibly damaging
increase in the temperature of the heating element. Damage is known as boiling
burnout. Under these circumstances, the region CD of Figure 3.3 is not
obtained.
Boiling
heat transfer coefficients depend on both the physical character of the liquid
and the nature of the heating surface. Through the agencies of wet-ting,
roughness, and contamination, the latter greatly influences the formation,
growth, and disengagement of bubbles in the nucleate boiling regime. There is,
at present, no reliable method of estimating the boiling coefficients of heat
transfer from the physical properties of the system. Coefficients, as shown for
water in Figure 3.3, are large, and higher resistances elsewhere will often
limit the rate at which heat can be transferred through a system as a whole.
Related Topics
