Rate, Rate Constants and Orders of Reactions
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Pharmacokinetics Basic Considerations
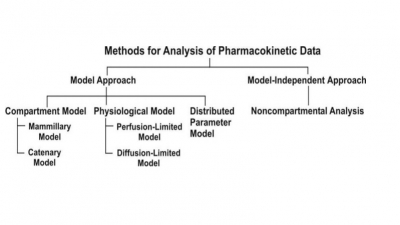
Pharmacokinetics is the mathematical analysis of processes of ADME.
Rate, Rate Constants and Orders of Reactions
Pharmacokinetics is the mathematical analysis of processes of ADME. The movement of drug
molecules from the site of application to the systemic circulation, through
various barriers, their conversion into another chemical form and finally their
exit out of the body can be expressed mathematically by the rate at which they
proceed, the order of such processes and the rate constants.
The velocity with which a reaction or a process occurs is called as its rate. The manner in which the
concentration of drug (or reactants) influences the rate of reaction or process
is called as the order of reaction or
order of process. Consider the following chemical reaction:
Drug A → Drug
B (8.1)
The rate of forward reaction is expressed as –
-dA/dt (8.2)
Negative sign indicates that the concentration of
drug A decreases with time t. As the reaction proceeds, the concentration of
drug B increases and the rate of reaction can also be expressed as:
dB/dt
(8.3)
Experimentally, the rate of reaction is determined by
measuring the decrease in concentration of drug A with time t.
If C is the concentration of drug A, the rate of
decrease in C of drug A as it is changed to B can be described by a general
expression as a function of time t.
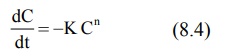
where, K = rate constant
n = order of reaction
If n = 0, its a zero-order process, if n = 1, it is
a first-order process and so on. The three commonly encountered rate processes
in a physiological system are —
·
Zero-order process
·
First-order process
·
Mixed-order process.
The pharmacokinetics of most drugs can be
adequately described by zero- and first-order processes of which the latter are
more important.
Related Topics