Regulation of Gluconeogenesis
| Home | | Biochemistry |Chapter: Biochemistry : Gluconeogenesis
The moment-to-moment regulation of gluconeogenesis is determined primarily by the circulating level of glucagon and by the availability of gluconeogenic substrates.
REGULATION OF GLUCONEOGENESIS
The moment-to-moment regulation of gluconeogenesis is determined primarily by the circulating level of glucagon and by the availability of gluconeogenic substrates. In addition, slow adaptive changes in enzyme activity result from an alteration in the rate of enzyme synthesis or degradation or both. [Note: Hormonal control of the glucoregulatory system is presented in Chapter 23.]
A. Glucagon
This peptide hormone
from the a cells of pancreatic islets stimulates gluconeogenesis by three
mechanisms.
1. Changes in allosteric effectors: Glucagon lowers the level of
fructose 2,6-bisphosphate, resulting in activation of fructose
1,6-bisphosphatase and inhibition of PFK-1, thus favoring gluconeogenesis over
glycolysis (see Figure 10.5). [Note: See for the role of fructose
2,6-bisphosphate in the regulation of glycolysis.]
2. Covalent modification of enzyme activity: Glucagon binds its G
protein–coupled receptor and, via an elevation in cyclic AMP (cAMP) level and
cAMP-dependent protein kinase activity, stimulates the conversion of hepatic PK
to its inactive (phosphorylated) form. This decreases the conversion of PEP to
pyruvate, which has the effect of diverting PEP to the synthesis of glucose
(Figure 10.8).
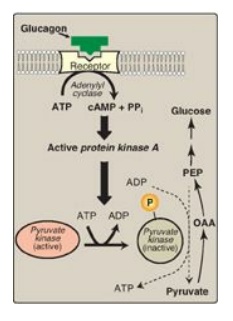
Figure 10.8 Covalent modification of pyruvate kinase results in inactivation of the enzyme. [Note: Only the hepatic isozyme is subject to covalent regulation.] OAA = oxaloacetate; PEP = phosphoenolpyruvate; cAMP = cyclic AMP; PPi = pyrophosphate; P = phosphate.
3. Induction of enzyme synthesis: Glucagon increases the
transcription of the gene for PEP-carboxykinase, thereby increasing the
availability of this enzyme as levels of its substrate rise during fasting.
[Note: Glucocorticoids also increase expression of the gene, whereas insulin
decreases expression.]
B. Substrate availability
The availability of
gluconeogenic precursors, particularly glucogenic amino acids, significantly
influences the rate of glucose synthesis. Decreased levels of insulin favor
mobilization of amino acids from muscle protein and provide the carbon
skeletons for gluconeogenesis. The ATP and NADH coenzymes-cosubstrates required
for gluconeogenesis are primarily provided by the catabolism of fatty acids.
C. Allosteric activation by acetyl coenzyme A
Allosteric activation
of hepatic pyruvate carboxylase by acetyl CoA occurs during fasting. As a
result of increased lipolysis in adipose tissue, the liver is flooded with
fatty acids. The rate of formation of acetyl CoA by β-oxidation of these fatty
acids exceeds the capacity of the liver to oxidize it to CO2 and H2O.
As a result, acetyl CoA accumulates and activates pyruvate carboxylase. [Note:
Acetyl CoA inhibits the PDH complex. Thus, this single compound can divert
pyruvate toward gluconeogenesis and away from the TCA cycle (Figure 10.9).]
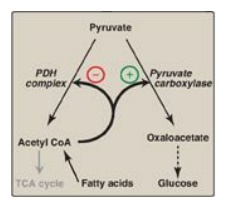
Figure 10.9 Acetyl coenzyme A (CoA) diverts pyruvate away from oxidation and toward gluconeogenesis. PDH = pyruvate dehydrogenase; TCA = tricarboxylic acid.
D. Allosteric inhibition by adenosine monophosphate
Fructose 1,6-bisphosphatase is inhibited by AMP—a compound that activates PFK-1. This results in a reciprocal regulation of glycolysis and gluconeogenesis seen previously with fructose 2,6-bisphosphate. [Note: Elevated AMP, thus, stimulates pathways that oxidize nutrients to provide energy for the cell.]
Related Topics
