Reactions Unique to Gluconeogenesis
| Home | | Biochemistry |Chapter: Biochemistry : Gluconeogenesis
Seven glycolytic reactions are reversible and are used in the synthesis of glucose from lactate or pyruvate. However, three of the reactions are irreversible and must be circumvented by four alternate reactions that energetically favor the synthesis of glucose.
REACTIONS UNIQUE TO GLUCONEOGENESIS
Seven glycolytic
reactions are reversible and are used in the synthesis of glucose from lactate
or pyruvate. However, three of the reactions are irreversible and must be
circumvented by four alternate reactions that energetically favor the synthesis
of glucose. These reactions, unique to gluconeogenesis, are described below.
A. Carboxylation of pyruvate
The first “roadblock”
to overcome in the synthesis of glucose from pyruvate is the irreversible
conversion in glycolysis of PEP to pyruvate by pyruvate kinase (PK). In
gluconeogenesis, pyruvate is first carboxylated by pyruvate carboxylase to OAA,
which is then converted to PEP by the action of PEP-carboxykinase (Figure
10.3).
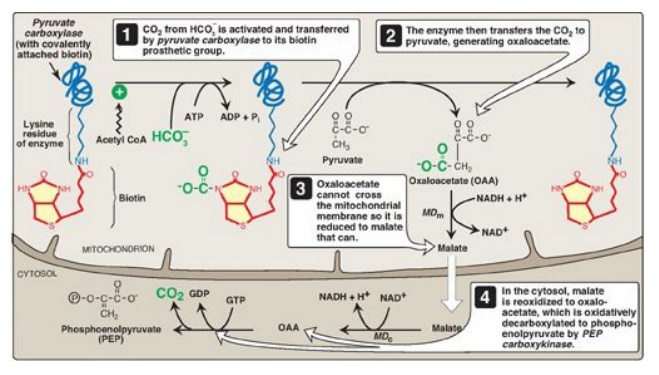
Figure 10.3 Carboxylation of pyruvate to OAA, followed by reduction of OAA to malate for transfer to the cystol and subsequent decarboxylation to PEP. [Note: OAA can also be converted to PEP or aspartate for transfer to the cytosol.] MDm = mitochondrial malate dehydrogenase; MDc = cytosolic malate dehydrogenase.
1. Biotin, a coenzyme: Pyruvate carboxylase requires
biotin covalently bound to the ε-amino group of a lysine residue in the enzyme
(see Figure 10.3). Hydrolysis of ATP drives the formation of an enzyme–biotin–CO2
intermediate, which subsequently carboxylates pyruvate to form OAA. [Note: HCO3–
is the source of the CO2.] The pyruvate carboxylase reaction occurs
in the mitochondria of liver and kidney cells and has two purposes: to provide
an important substrate for gluconeogenesis and to provide OAA that can
replenish the TCA cycle intermediates that may become depleted, depending on
the synthetic needs of the cell. Muscle cells also contain pyruvate carboxylase
but use the OAA produced only for the replenishment (anaplerotic) purpose and
do not synthesize glucose.
Pyruvate carboxylase is one of several carboxylases
that require biotin. Others include acetyl CoA carboxylase, propionyl CoA
carboxylase, and methylcrotonyl CoA carboxylase.
2. Allosteric regulation: Pyruvate carboxylase is allosterically activated by acetyl CoA. Elevated levels of acetyl CoA in mitochondria signal a metabolic state in which the increased synthesis of OAA is required. For example, this occurs during fasting, when OAA is used for the synthesis of glucose by gluconeogenesis in the liver and kidney. Conversely, at low levels of acetyl CoA, pyruvate carboxylase is largely inactive, and pyruvate is primarily oxidized by the PDH complex to produce acetyl CoA that can be further oxidized by the TCA cycle.
B. Transport of oxaloacetate to the cytosol
OAA must be converted
to PEP for gluconeogenesis to continue. The enzyme that catalyzes this reaction
is found in both the mitochondria and the cytosol in humans. The PEP generated
in the mitochondria is transported to the cytosol by a specific transporter,
whereas that generated in the cytosol requires the transport of OAA from the
mitochondria to the cytosol. However, OAA is unable to be transported across
the inner mitochondrial membrane, so it must first be reduced to malate by
mitochondrial malate dehydrogenase (MD). Malate can be transported from the
mitochondria to the cytosol, where it is reoxidized to OAA by cytosolic MD as
nicotinamide adenine dinucleotide (NAD+) is reduced (see Figure
10.3). The NADH produced is used in the reduction of 1,3-bisphosphoglycerate to
glyceraldehyde 3-phosphate, a step common to both glycolysis and
gluconeogenesis. [Note: OAA also can be converted to aspartate, which is
transported out of the mitochondria.]
C. Decarboxylation of cytosolic oxaloacetate
OAA is decarboxylated
and phosphorylated to PEP in the cytosol by PEP-carboxykinase (also referred to
as PEPCK). The reaction is driven by hydrolysis of guanosine triphosphate
([GTP] see Figure 10.3). The combined actions of pyruvate carboxylase and
PEP-carboxykinase provide an energetically favorable pathway from pyruvate to
PEP. PEP is then acted on by the reactions of glycolysis running in the reverse
direction until it becomes fructose 1,6-bisphosphate.
The pairing of carboxylation with decarboxylation, as seen in gluconeogenesis, drives reactions that would otherwise be energetically unfavorable. A similar strategy is used in fatty acid synthesis.
D. Dephosphorylation of fructose 1,6-bisphosphate
Hydrolysis of fructose
1,6-bisphosphate by fructose 1,6-bisphos-phatase, found in liver and kidney,
bypasses the irreversible phosphofructokinase-1 (PFK-1) reaction, and provides
an energetically favorable pathway for the formation of fructose 6-phosphate
(Figure 10.4). This reaction is an important regulatory site of
gluconeogenesis.
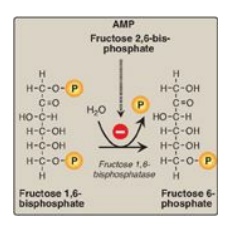
Figure 10.4 Dephosphorylation of fructose 1,6- bisphosphate. AMP = adenosine monophosphate; P = phosphate.
1. Regulation by energy levels within the cell: Fructose 1,6-bisphosphatase is inhibited by elevated levels of adenosine monophosphate (AMP), which signal an “energy-poor” state in the cell. Conversely, high levels of ATP and low concentrations of AMP stimulate gluconeogenesis, an energy-requiring pathway.
2. Regulation by fructose 2,6-bisphosphate: Fructose 1,6-bisphos-phatase is
inhibited by fructose 2,6-bisphosphate, an allosteric effector whose
concentration is influenced by the insulin to glucagon ratio: when glucagon is
high, the effector is not made and, thus, the phosphatase is active. (Figure
10.5). [Note: The signals that inhibit (low energy, high fructose
2,6-bisphosphate) or activate (high energy, low fructose 2,6-bisphosphate)
gluconeogenesis have the opposite effect on glycolysis, providing reciprocal
control of the pathways that synthesize and oxidize glucose
.]
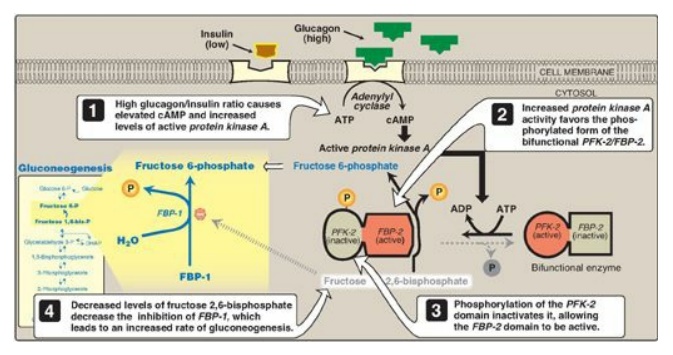
Figure 10.5 Effect of elevated glucagon on the intracellular concentration of fructose 2,6-bisphosphate in the liver. cAMP = cyclic AMP; PFK-2 = phosphofructokinase-2; FBP-2 = fructose 2,6-bisphosphatase; FBP-1 = fructose 1,6-bisphosphatase; P = phosphate.
E. Dephosphorylation of glucose 6-phosphate
Hydrolysis of glucose
6-phosphate by glucose 6-phosphatase bypasses the irreversible
hexokinase/glucokinase reaction and provides an energetically favorable pathway
for the formation of free glucose (Figure 10.6). Liver and kidney are the only
organs that release free glucose from glucose 6-phosphate. This process
actually requires a complex of two proteins: glucose 6-phosphate translocase,
which transports glucose 6-phosphate across the endoplasmic reticular (ER)
membrane, and the enzyme glucose 6-phosphatase (found only in gluconeogenic
cells), which removes the phosphate, producing free glucose (see Figure 10.6).
[Note: These ER-membrane proteins are also required for the final step of
glycogen degradation. Type Ia and lb glycogen storage disease, caused by
deficiencies in the phosphatase and the transferase, respectively, are
characterized by severe fasting hypoglycemia, because free glucose is unable to
be produced from either gluconeogenesis or glycogenolysis.] Specific glucose
transporters (GLUTs) are responsible for moving free glucose into the cytosol
and then into blood. [Note: Glucose 6-phosphate translocase moves inorganic
phosphate out of the ER as it moves glucose 6-phosphate in.]
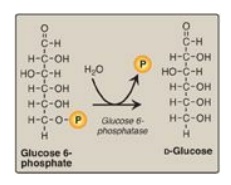
Figure 10.6 Dephosphorylation of glucose 6-phosphate allows release of free glucose from the liver and kidney into blood. P = phosphate.
F. Summary of the reactions of glycolysis and gluconeogenesis
Of the 11 reactions
required to convert pyruvate to free glucose, 7 are catalyzed by reversible
glycolytic enzymes (Figure 10.7). The irreversible reactions of glycolysis
catalyzed by hexokinase/glucokinase, PFK-1, and PK are circumvented by glucose
6-phosphatase, fructose 1,6-bisphosphatase, and pyruvate
carboxylase/PEP-carboxykinase. In gluconeogenesis, the equilibria of the 7
reversible reactions of glycolysis are pushed in favor of glucose synthesis as
a result of the essentially irreversible formation of PEP, fructose
6-phosphate, and glucose catalyzed by the gluconeogenic enzymes. [Note: The
stoichiometry of gluconeogenesis from pyruvate couples the cleavage of six
high-energy phosphate bonds and the oxidation of two NADH with the formation of
each molecule of glucose (see Figure 10.7).]
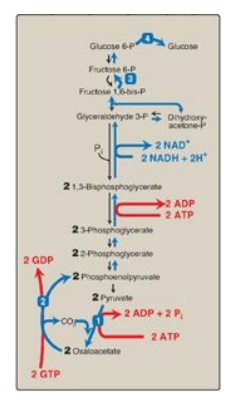
Figure 10.7 Summary of the
reactions of glycolysis and gluconeogenesis, showing the energy requirements of
gluconeogenesis. The numbered reactions are unique to gluconeogenesis. P =
phosphate; GDP = guanosine diphosphate; GTP = guanosine triphosphate; NAD(H) =
nicotinamide adenine dinucleotide.
Related Topics
