Synthesis of Glycogen (Glycogenesis)
| Home | | Biochemistry |Chapter: Biochemistry : Glycogen Metabolism
Glycogen is synthesized from molecules of α-D-glucose. The process occurs in the cytosol and requires energy supplied by ATP (for the phosphorylation of glucose) and uridine triphosphate (UTP).
SYNTHESIS OF GLYCOGEN (GLYCOGENESIS)
Glycogen is synthesized
from molecules of α-D-glucose. The process occurs in the cytosol and requires
energy supplied by ATP (for the phosphorylation of glucose) and uridine
triphosphate (UTP).
A. Synthesis of uridine diphosphate glucose
α-D-Glucose attached to
uridine diphosphate (UDP) is the source of all the glucosyl residues that are
added to the growing glycogen molecule. UDP-glucose (Figure 11.4) is
synthesized from glucose 1-phosphate and UTP by UDP-glucose pyrophosphorylase
(Figure 11.5). Pyrophosphate (PPi), the second product of the reaction, is
hydrolyzed to two inorganic phosphates (Pi) by pyrophosphatase. The hydrolysis
is exergonic, ensuring that the UDP-glucose pyrophosphorylase reaction proceeds
in the direction of UDP-glucose production. [Note: Glucose 1-phosphate is generated
from glucose 6-phosphate by phosphoglucomutase. Glucose 1,6-bisphosphate is an
obligatory intermediate in this reversible reaction (Figure 11.6).]
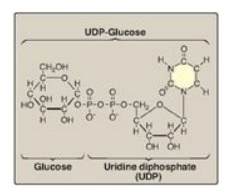
Figure 11.4 The structure of UDP-glucose, a nucleotide sugar.
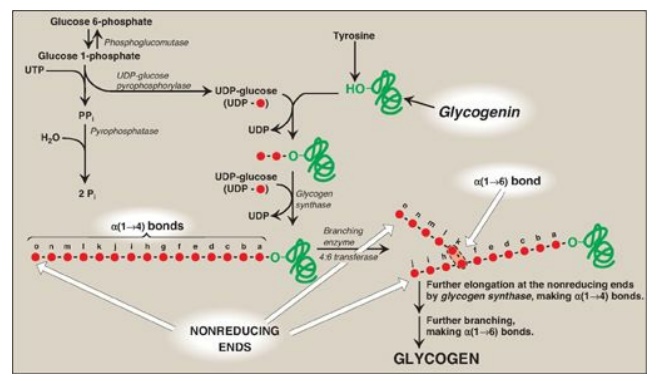
Figure 11.5 Glycogen synthesis. UTP = uridine triphosphate; UDP = uridine diphosphate; PPi = pyrophosphate; Pi = inorganic phosphate.
B. Synthesis of a primer to initiate glycogen synthesis
Glycogen synthase makes the α(1→4) linkages in glycogen. This enzyme cannot initiate chain synthesis using free glucose as an acceptor of a molecule of glucose from UDP-glucose. Instead, it can only elongate already existing chains of glucose and, therefore, requires a primer. A fragment of glycogen can serve as a primer in cells whose glycogen stores are not totally depleted. In the absence of a glycogen fragment, a protein called glycogenin can serve as an acceptor of glucose residues from UDP-glucose (see Figure 11.5). The side-chain hydroxyl group of a specific tyrosine in the protein serves as the site at which the initial glucosyl unit is attached. Because the reaction is catalyzed by glycogenin itself via autoglucosylation, glycogenin is an enzyme. Glycogenin then catalyzes the transfer of the next few molecules of glucose from UDP-glucose, producing a short, α(1→4)-linked glucosyl chain. This short chain serves as a primer that is able to be elongated by glycogen synthase as described below [Note: Glycogenin stays associated with and forms the core of a glycogen granule.]
C. Elongation of glycogen chains by glycogen synthase
Elongation of a
glycogen chain involves the transfer of glucose from UDP-glucose to the
nonreducing end of the growing chain, forming a new glycosidic bond between the
anomeric hydroxyl group of carbon 1 of the activated glucose and carbon 4 of
the accepting glucosyl residue (see Figure 11.5). [Note: The nonreducing end of
a carbohydrate chain is one in which the anomeric carbon of the terminal sugar
is linked by a glycosidic bond to another compound, making the terminal sugar
nonreducing.] The enzyme responsible for making the α(1→4) linkages in glycogen
is glycogen synthase. [Note: The UDP released when the new α(1→4) glycosidic
bond is made can be phosphorylated to UTP by nucleoside diphosphate kinase (UDP
+ ATP UTP + ADP;]
D. Formation of branches in glycogen
If no other synthetic enzyme acted on the chain, the resulting structure would be a linear (unbranched) chain of glucosyl residues attached by α(1→4) linkages. Such a compound is found in plant tissues and is called amylose. In contrast, glycogen has branches located, on average, eight glucosyl residues apart, resulting in a highly branched, tree-like structure (see Figure 11.3) that is far more soluble than the unbranched amylose. Branching also increases the number of nonreducing ends to which new glucosyl residues can be added (and also, as described later, from which these residues can be removed), thereby greatly accelerating the rate at which glycogen synthesis can occur and dramatically increasing the size of the glycogen molecule.
1. Synthesis of branches: Branches are made by the action of
the branching enzyme, amylo-α(1→4)→α(1→6)-transglucosidase. This enzyme removes
a set of six to eight glucosyl residues from the nonreducing end of the
glycogen chain, breaking an α(1→4) bond to another residue on the chain, and
attaches it to a non-terminal glucosyl residue by an α(1→6) linkage, thus
functioning as a 4:6 transferase. The resulting new, nonreducing end (see “j”
in Figure 11.5), as well as the old nonreducing end from which the six to eight
residues were removed (see “o” in Figure 11.5), can now be further elongated by
glycogen synthase.
2. Synthesis of additional branches: After elongation of these two ends has been accomplished, their terminal six to eight glucosyl residues can be removed and used to make additional branches.
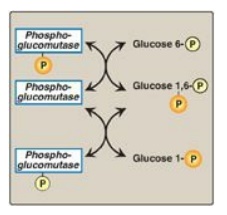
Figure 11.6 Interconversion of glucose 6-phosphate and glucose 1-phosphate by phosphoglucomutase. P = phosphate.
Related Topics
