Fructose Metabolism
| Home | | Biochemistry |Chapter: Biochemistry : Metabolism of Monosaccharides and Disaccharides
About 10% of the calories comprising the Western diet are supplied by fructose (approximately 55 g/day).
FRUCTOSE METABOLISM
About 10% of the
calories comprising the Western diet are supplied by fructose (approximately 55
g/day). The major source of fructose is the disaccharide sucrose, which, when
cleaved in the intestine, releases equimolar amounts of fructose and glucose.
Fructose is also found as a free monosaccharide in many fruits, in honey, and
in high-fructose corn syrup (typically, 55% fructose/45% glucose), which is
used to sweeten soft drinks and many foods. Fructose transport into cells is
not insulin dependent (unlike that of glucose into certain tissues;), and, in
contrast to glucose, fructose does not promote the secretion of insulin.
A. Phosphorylation of fructose
For fructose to enter
the pathways of intermediary metabolism, it must first be phosphorylated
(Figure 12.2). This can be accomplished by either hexokinase or fructokinase.
Hexokinase phosphorylates glucose in most cells of the body, and several
additional hexoses can serve as substrates for this enzyme. However, it has a
low affinity (that is, a high Michaelis constant [Km];) for fructose.
Therefore, unless the intracellular concentration of fructose becomes unusually
high, the normal presence of saturating concentrations of glucose means that
little fructose is phosphorylated by hexokinase. Fructokinase provides the
primary mechanism for fructose phosphorylation (see Figure 12.2). The enzyme
has a low Km for fructose and a high Vmax (or, maximal velocity;). It is found
in the liver (which processes most of the dietary fructose), kidney, and the
small intestinal mucosa and converts fructose to fructose 1-phosphate, using
adenosine triphosphate (ATP) as the phosphate donor. [Note: These three tissues
also contain aldolase B, discussed in section B.]
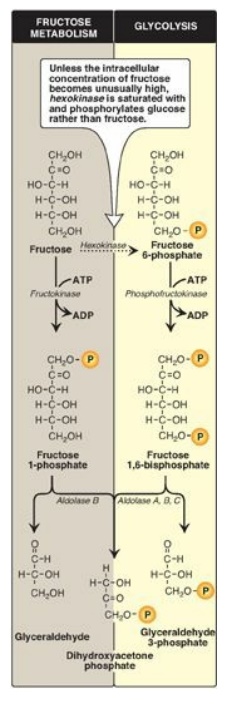
Figure 12.2 Phosphorylation products of fructose and their cleavage. P = phosphate; ADP = adenosine diphosphate.
B. Cleavage of fructose 1-phosphate
Fructose 1-phosphate is not phosphorylated to fructose 1,6-bisphos-phate as is fructose 6-phosphate but is cleaved by aldolase B (also called fructose 1-phosphate aldolase) to dihydroxyacetone phosphate (DHAP) and glyceraldehyde. [Note: Humans express three aldolases, A, B and C, the products of three different genes. Aldolase A (found in most tissues), aldolase B (in liver, kidney, and small intestine), and aldolase C (in brain) all cleave fructose 1,6-bisphosphate produced during glycolysis to DHAP and glyceraldehyde 3-phosphate, but only aldolase B cleaves fructose 1-phosphate.] DHAP can directly enter glycolysis or gluconeogenesis, whereas glyceraldehyde can be metabolized by a number of pathways, as illustrated in Figure 12.3.
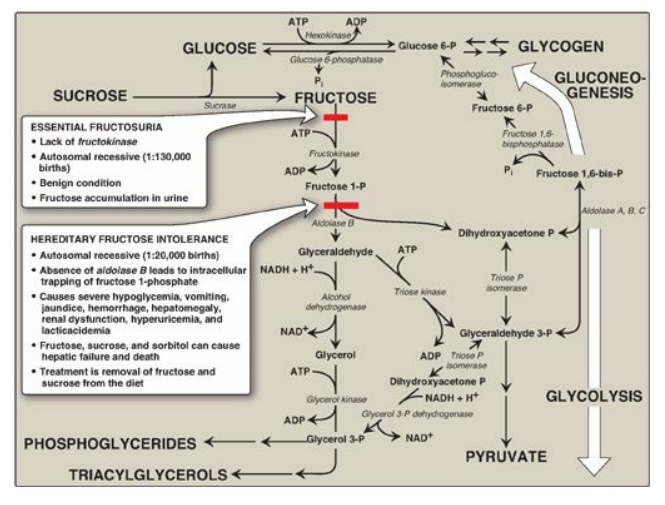
Figure 12.3 Summary of fructose metabolism. P = phosphate; Pi = inorganic phosphate; NAD(H) = nicotinamide adenine dinucleotide; ADP = adenosine diphosphate.
C. Kinetics of fructose metabolism
The rate of fructose
metabolism is more rapid than that of glucose because the trioses formed from
fructose 1-phosphate bypass phosphofructokinase-1, the major rate-limiting step
in glycolysis.
D. Disorders of fructose metabolism
A deficiency of one of
the key enzymes required for the entry of fructose into metabolic pathways can
result in either a benign condition as a result of fructokinase deficiency
(essential fructosuria) or a severe disturbance of liver and kidney metabolism
as a result of aldolase B deficiency (hereditary fructose intolerance [HFI]),
which is estimated to occur in 1:20,000 live births (see Figure 12.3). The
first symptoms of HFI appear when a baby is weaned from milk and begins to be
fed food containing sucrose or fructose. Fructose 1-phosphate accumulates,
resulting in a drop in the level of inorganic phosphate (Pi) and, therefore, of
ATP production. As ATP falls, adenosine monophosphate (AMP) rises. The AMP is
degraded, causing hyperuricemia (and lactic acidosis;). The decreased
availability of hepatic ATP affects gluconeogenesis (causing hypoglycemia with
vomiting) and protein synthesis (causing a decrease in blood clotting factors
and other essential proteins). Kidney function may also be affected. [Note: The
drop in Pi also inhibits glycogenolysis.] Diagnosis of HFI can be made on the
basis of fructose in the urine, enzyme assay using liver cells, or by DNA-based
testing (see Chapter 33) . Aldolase B deficiency is part of the newborn screening
panel. With HFI, sucrose, as well as fructose, must be removed from the diet to
prevent liver failure and possible death. Individuals with HFI display an
aversion to sweets and, consequently, have an absence of dental caries.
E. Conversion of mannose to fructose 6-phosphate
Mannose, the C-2 epimer of glucose, is an important component of glycoproteins. Hexokinase phosphorylates mannose, producing mannose 6-phosphate, which, in turn, is reversibly isomerized to fructose 6-phosphate by phosphomannose isomerase. [Note: There is little mannose in dietary carbohydrates. Most intracellular mannose is synthesized from fructose or is preexisting mannose produced by the degradation of structural carbohydrates and salvaged by hexokinase.]
F. Conversion of glucose to fructose via sorbitol
Most sugars are rapidly
phosphorylated following their entry into cells. Therefore, they are trapped
within the cells, because organic phosphates cannot freely cross membranes
without specific transporters. An alternate mechanism for metabolizing a
monosaccharide is to convert it to a polyol (sugar alcohol) by the reduction of
an aldehyde group, thereby producing an additional hydroxyl group.
1. Synthesis of sorbitol: Aldose reductase reduces glucose,
producing sorbitol (glucitol; Figure 12.4). This enzyme is found in many
tissues, including the lens, retina, Schwann cells of peripheral nerves, liver,
kidney, placenta, red blood cells, and cells of the ovaries and seminal
vesicles. In cells of the liver, ovaries, and seminal vesicles, there is a
second enzyme, sorbitol dehydrogenase, which can oxidize the sorbitol to
produce fructose (see Figure 12.4). The two-reaction pathway from glucose to
fructose in the seminal vesicles benefits sperm cells, which use fructose as a
major carbohydrate energy source. The pathway from sorbitol to fructose in the
liver provides a mechanism by which any available sorbitol is converted into a
substrate that can enter glycolysis or gluconeogenesis.
2. Effect of hyperglycemia on sorbitol metabolism: Because insulin is not required
for the entry of glucose into the cells listed in the previous paragraph, large
amounts of glucose may enter these cells during times of hyperglycemia (for
example, in uncontrolled diabetes). Elevated intracellular glucose
concentrations and an adequate supply of reduced nicotinamide adenine dinucleotide
phosphate (NADPH) cause aldose reductase to produce a significant increase in
the amount of sorbitol, which cannot pass efficiently through cell membranes
and, in turn, remains trapped inside the cell (see Figure 12.4). This is
exacerbated when sorbitol dehydrogenase is low or absent (for example, in
retina, lens, kidney, and nerve cells). As a result, sorbitol accumulates in
these cells, causing strong osmotic effects and, therefore, cell swelling as a
result of water retention. Some of the pathologic alterations associated with
diabetes can be attributed, in part, to this phenomenon, including cataract
formation, peripheral neuropathy, and microvascular problems leading to
nephropathy and retinopathy. (See : for a discussion of the complications of
diabetes.) [Note: Use of NADPH in the aldose reductase reaction decreases the
generation of reduced glutathione, an important antioxidant, and may be related
to diabetic complications.]
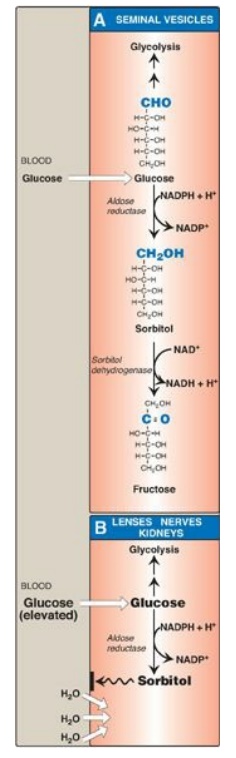
Figure 12.4 Sorbitol
metabolism. NAD(H) = nicotinamide adenine dinucleotide; NADP(H) = nicotinamide
adenine dinucleotide phosphate.
Related Topics
