Uses of NADPH
| Home | | Biochemistry |Chapter: Biochemistry : Pentose Phosphate Pathway and Nicotinamide Adenine Dinucleotide Phosphate
The coenzyme NADPH differs from nicotinamide adenine dinucleotide (NADH) only by the presence of a phosphate group on one of the ribose units.
USES OF NADPH
The coenzyme NADPH
differs from nicotinamide adenine dinucleotide (NADH) only by the presence of a
phosphate group on one of the ribose units (Figure 13.4). This seemingly small
change in structure allows NADPH to interact with NADPH-specific enzymes that
have unique roles in the cell. For example, in the cytosol of hepatocytes the
steady-state ratio of NADP+/NADPH is approximately 0.1, which favors the use of
NADPH in reductive biosynthetic reactions. This contrasts with the high ratio
of NAD+/NADH (approximately 1000), which favors an oxidative role
for NAD+. This section summarizes some important NADP+
and NADPH-specific functions in reductive biosynthesis and detoxification
reactions.
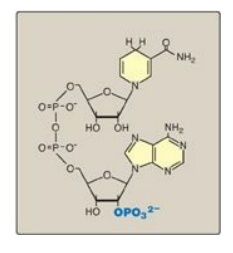
Figure 13.4 Structure of reduced nicotinamide adenine dinucleotide phosphate (NADPH).
A. Reductive biosynthesis
NADPH can be thought of
as a high-energy molecule, much in the same way as NADH. However, the electrons
of NADPH are destined for use in reductive biosynthesis, rather than for
transfer to oxygen as is the case with NADH. Thus, in the metabolic
transformations of the pentose phosphate pathway, part of the energy of glucose
6-phosphate is conserved in NADPH, a molecule with a negative reduction
potential, that, therefore, can be used in reactions requiring an electron
donor, such as fatty acid and steroid synthesis.
B. Reduction of hydrogen peroxide
Hydrogen peroxide (H2O2)
is one of a family of reactive oxygen species (ROS) that are formed from the
partial reduction of molecular oxygen (Figure 13.5A). These compounds are
formed continuously as byproducts of aerobic metabolism, through reactions with
drugs and environmental toxins, or when the level of antioxidants is
diminished, all creating the condition of oxidative stress. The highly reactive
oxygen intermediates can cause serious chemical damage to DNA, proteins, and
unsaturated lipids and can lead to cell death. ROS have been implicated in a
number of pathologic processes, including reperfusion injury, cancer,
inflammatory disease, and aging. The cell has several protective mechanisms
that minimize the toxic potential of these compounds.
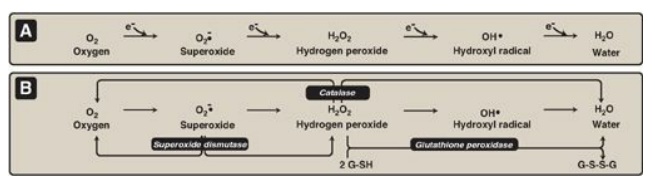
Figure 13.5 A. Formation of reactive intermediates from molecular oxygen. e- = electrons. B. Actions of antioxidant enzymes. G-SH = reduced glutathione; G-S-S-G = oxidized glutathione. (See Figure 13.6B for the regeneration of G-SH.)
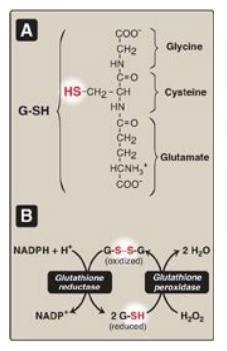
Figure 13.6 A. Structure of
reduced glutathione (G-SH). [Note: Glutamate is linked to cysteine through a
γ-carboxyl, rather than an α-carboxyl.] B. Glutathione-mediated reduction of
hydrogen peroxide (H2O2) by reduced nicotinamide adenine
dinucleotide phosphate (NADPH). G-S-S-G = oxidized glutathione.
1. Enzymes that catalyze antioxidant reactions: Reduced glutathione (G-SH), a
tripeptide-thiol (γ-glutamylcysteinylglycine) present in most cells, can
chemically detoxify H2O2 (Figure 13.5B). This reaction,
catalyzed by the selenium-containing glutathione peroxidase, forms oxidized
glutathione (G-S-S-G), which no longer has protective properties. The cell
regenerates G-SH in a reaction catalyzed by glutathione reductase, using NADPH
as a source of reducing equivalents. Thus, NADPH indirectly provides electrons
for the reduction of H2O2 (Figure 13.6). [Note: RBCs are
totally dependent on the pentose phosphate pathway for their supply of NADPH
because, unlike other cell types, RBCs do not have an alternate source for this
essential coenzyme.] Additional enzymes, such as superoxide dismutase and
catalase, catalyze the conversion of other reactive oxygen intermediates to
harmless products (see Figure 13.5B). As a group, these enzymes serve as a
defense system to guard against the toxic effects of ROS.
2. Antioxidant chemicals: A number of intracellular reducing
agents, such as ascorbate, vitamin E, and β-carotene, are able to reduce and,
thereby, detoxify reactive oxygen intermediates in the laboratory. Consumption
of foods rich in these antioxidant compounds has been correlated with a reduced
risk for certain types of cancers as well as decreased frequency of certain
other chronic health problems. Therefore, it is tempting to speculate that the
effects of these compounds are, in part, an expression of their ability to
quench the toxic effect of ROS. However, clinical trials with antioxidants as
dietary supplements have failed to show clear beneficial effects. In the case
of dietary supplementation with β-carotene, the rate of lung cancer in smokers
increased rather than decreased. Thus, the health-promoting effects of dietary
fruits and vegetables likely reflect a complex interaction among many naturally
occurring compounds, which has not been duplicated by consumption of isolated
antioxidant compounds.
C. Cytochrome P450 monooxygenase system
Monooxygenases
(mixed-function oxidases) incorporate one atom from molecular oxygen into a
substrate (creating a hydroxyl group), with the other atom being reduced to
water. In the cytochrome P450 monooxygenase system, NADPH provides the reducing
equivalents required by this series of reactions (Figure 13.7). This system
performs different functions in two separate locations in cells. The overall
reaction catalyzed by a cytochrome P450 enzyme is:
R-H + O2 +
NADPH + H+ → R-OH + H2O + NADP+
where R may be a
steroid, drug, or other chemical. [Note: Cyto-chrome P450 (CYP) enzymes are
actually a superfamily of related, heme-containing monooxygenases that participate
in a broad variety of reactions. The P450 in the name reflects the absorbance
at 450 nm by the protein.]
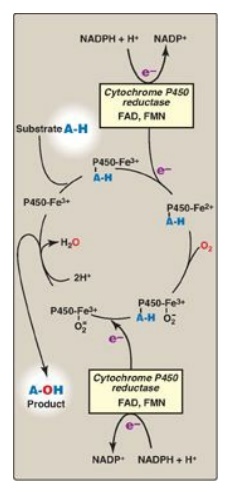
Figure 13.7 Cytochrome P450 monooxygenase cycle (simplified). Electrons (e-) move from NADPH to FAD to FMN of the reductase and then to the heme iron (Fe) of the P450 enzyme. [Note: In the mitochondrial system, electrons move from FAD to an ironsulfur protein and then to the P450 enzyme.] FAD = flavin adenine dinucleotide; FMN = flavin mononucleotide; NADPH = reduced nicotinamide adenine dinucleotide phosphate.
1. Mitochondrial system: An important function of the
cytochrome P450 monooxygenase system found associated with the inner
mitochondrial membrane is the biosynthesis of steroid hormones. In
steroidogenic tissues, such as the placenta, ovaries, testes, and adrenal
cortex, it is used to hydroxylate intermediates in the conversion of
cholesterol to steroid hormones, a process that makes these hydrophobic
compounds more water soluble. The liver uses this same system in bile acid
synthesis and the hydroxylation of cholecalciferol to 25-hydroxycholecalciferol
(vitamin D3;), and the kidney uses it to hydroxylate vitamin D3 to its
biologically active 1,25-dihydroxylated form.
2. Microsomal system: An extremely important function of the microsomal cytochrome P450 monooxygenase system found associated with the membrane of the smooth endoplasmic reticulum (particularly in the liver) is the detoxification of foreign compounds (xenobiotics). These include numerous drugs and such varied pollutants as petroleum products and pesticides. CYP enzymes of the microsomal system (for example, CYP3A4), can be used to hydroxylate these toxins. The purpose of these modifications is two-fold. First, it may itself activate or inactivate a drug and second, make a toxic compound more soluble, thereby facilitating its excretion in the urine or feces. Frequently, however, the new hydroxyl group will serve as a site for conjugation with a polar molecule, such as glucuronic acid, which will significantly increase the compound’s solubility. [Note: Polymorphisms in the genes for CYP enzymes can lead to differences in drug metabolism.]
D. Phagocytosis by white blood cells
Phagocytosis is the
ingestion by receptor-mediated endocytosis of microorganisms, foreign
particles, and cellular debris by cells such as neutrophils and macrophages
(monocytes). It is an important defense mechanism, particularly in bacterial
infections. Neutrophils and monocytes are armed with both oxygen-independent
and oxygen-dependent mechanisms for killing bacteria.
1. Oxygen-independent mechanism: Oxygen-independent mechanisms use
pH changes in phagolysosomes and lysosomal enzymes to destroy pathogens.
2. Oxygen-dependent system: Oxygen-dependent mechanisms include
the enzymes NADPH oxidase and myeloperoxidase (MPO) that work together in
killing bacteria (Figure 13.8). Overall, the MPO system is the most potent of
the bactericidal mechanisms. An invading bacterium is recognized by the immune
system and attacked by antibodies that bind it to a receptor on a phagocytic
cell. After internalization of the microorganism has occurred, NADPH oxidase,
located in the leukocyte cell membrane, is activated and reduces O2 from
the surrounding tissue to superoxide (O2-• ), a free
radical, as NADPH is oxidized. The rapid consumption of O2 that
accompanies formation of O2-.
is referred to as the “respiratory burst.” [Note: Active NADPH oxidase is a
membrane-associated complex containing a flavocytochrome plus additional
peptides that translocate from the cytoplasm upon activation of the leukocyte.
Electrons move from NADPH to O2 via flavin adenine nucleotide (FAD)
and heme, generating . Rare genetic deficiencies in NADPH oxidase cause chronic
granulomatous disease (CGD) characterized by severe, persistent infections and
the formation of granulomas (nodular areas of inflammation) that sequester the
bacteria that were not destroyed.] Next, O2-. is converted to H2O2 (a ROS), either
spontaneously or catalyzed by superoxide dismutase. In the presence of MPO, a
heme-containing lysosomal enzyme present within the phagolysosome, peroxide
plus chloride ions are converted to hypochlorous acid ([HOCl] the major
component of household bleach), which kills the bacteria. The peroxide can also
be partially reduced to the hydroxyl radical (OH•), a ROS, or be fully reduced
to water by catalase or glutathione peroxidase. [Note: Deficiencies i n MPO do
not confer increased susceptibility to infection because peroxide from NADPH
oxidase is bactericidal.]
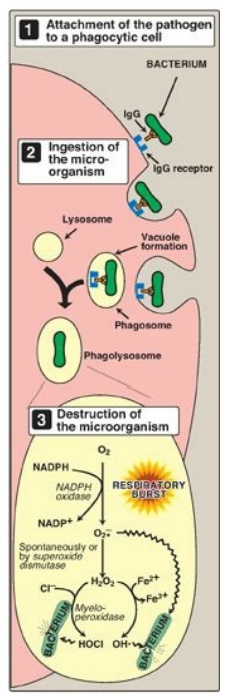
Figure 13.8 Phagocytosis and
the oxygendependent pathway of microbial killing. IgG = the antibody
immunoglobulin G; NADPH = reduced nicotinamide adenine dinucleotide phosphate; O2-• = superoxide; HOCl = hypochlorous acid; OH•
= hydroxyl radical.
E. Synthesis of nitric oxide
Nitric oxide (NO) is
recognized as a mediator in a broad array of biologic systems. NO is the
endothelium-derived relaxing factor, which causes vasodilation by relaxing
vascular smooth muscle. NO also acts as a neurotransmitter, prevents platelet
aggregation, and plays an essential role in macrophage function. NO has a very
short half-life in tissues (3–10 seconds) because it reacts with oxygen and
superoxide and then is converted into nitrates and nitrites including
peroxynitrite (O=NOO–), a reactive nitrogen species (RNS). [Note: NO is a free
radical gas that is often confused with nitrous oxide (N2O), the
“laughing gas” that is used as an anesthetic and is chemically stable.]
1. Nitric oxide synthase: Arginine, O2, and NADPH
are substrates for cytosolic NO synthase ([NOS] Figure 13.9). Flavin
mononucleotide (FMN), FAD, heme, and tetrahydrobiopterin are coenzymes, and NO
and citrulline are products of the reaction. Three NOS, each the product of a
different gene, have been identified. Two are constitutive (synthesized at a
constant rate), Ca2+–calmodulin-dependent enzymes. They are found
primarily in endothelium (eNOS) and neural tissue (nNOS) and constantly produce
very low levels of NO for vasodilation and neurotransmission. An inducible, Ca2+-independent
enzyme (iNOS) can be expressed in many cells, including macrophages and
neutrophils, as an early defense against pathogens. The specific inducers for
iNOS vary with cell type, and include proinflammatory cytokines, such as tumor
necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), and bacterial endotoxins
such as lipopolysaccharide (LPS). These compounds promote synthesis of iNOS,
which can result in large amounts of NO being produced over hours or even days.
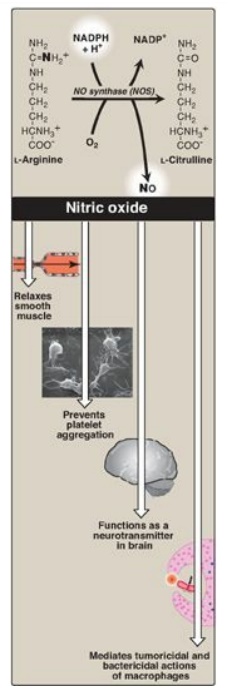
Figure 13.9 Synthesis and some of the actions of nitric oxide (NO). NADPH = reduced nicotinamide adenine dinucleotide phosphate. [Note: Flavin mononucleotide, flavin adenine dinucleotide, heme, and tetrahydrobiopterin are additional coenzymes required by NOS.]
2. Actions of nitric oxide on vascular endothelium: NO is an important mediator in the
control of vascular smooth muscle tone. NO is synthesized by eNOS in
endothelial cells and diffuses to vascular smooth muscle, where it activates
the cytosolic form of guanylate cyclase (also known as guanylyl cyclase) to
form cyclic guanosine monophosphate (cGMP). [Note: This reaction is analogous
to the formation of cyclic AMP by adenylate cyclase, except that this guanylate
cyclase is not membrane associated.] The resultant rise in cGMP causes
activation of protein kinase G, which phosphorylates Ca2+ channels,
causing decreased entry of Ca2+ into smooth muscle cells. This
decreases the calcium–calmodulin activation of myosin light-chain kinase,
thereby decreasing smooth muscle contraction and favoring relaxation.
Vasodilator nitrates, such as nitroglycerin, are metabolized to NO, which
causes relaxation of vascular smooth muscle and, therefore, lowers blood
pressure. Thus, NO can be envisioned as an endogenous nitrovasodilator. [Note:
NO is involved in penile erection. Sildenafil citrate, used in the treatment of
erectile dysfunction, inhibits the phosphodiesterase that inactivates cGMP.]
3. Role of nitric oxide in macrophage bactericidal activity: In macrophages, iNOS activity is normally low, but synthesis of the enzyme is significantly stimulated by bacterial LPS and by release of IFN-γ and TNF-α in response to the infection. Activated macrophages form CO2•- radicals that combine with NO to form intermediates that decompose, producing the highly bactericidal OH• radical.
4. Other functions of nitric oxide: NO is a potent inhibitor of platelet adhesion and aggregation (by activating the cGMP pathway). It is also characterized as a neurotransmitter in the central and peripheral nervous systems.
Related Topics
