Structure of Glycosaminoglycans
| Home | | Biochemistry |Chapter: Biochemistry : Glycosaminoglycans, Proteoglycans, and Glycoproteins
GAGs are long, unbranched, heteropolysaccharide chains composed of a repeating disaccharide unit [acidic sugar–amino sugar]n.
STRUCTURE OF GLYCOSAMINOGLYCANS
GAGs are long,
unbranched, heteropolysaccharide chains composed of a repeating disaccharide
unit [acidic sugar–amino sugar]n (Figure 14.1). [Note: A single exception is
keratan sulfate, which contains galactose rather than an acidic sugar.] The
amino sugar is either D-glucosamine or D-galactosamine, in which the amino
group is usually acetylated, thus eliminating its positive charge. The amino
sugar may also be sulfated on carbon 4 or 6 or on a nonacetylated nitrogen. The
acidic sugar is either D-glucuronic acid or its C-5 epimer L-iduronic acid
(Figure 14.2). These acidic sugars contain carboxyl groups that are negatively
charged at physiologic pH and, together with the sulfate groups, give GAGs
their strongly negative nature.
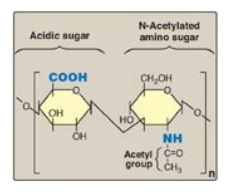
Figure 14.1 Repeating disaccharide unit.
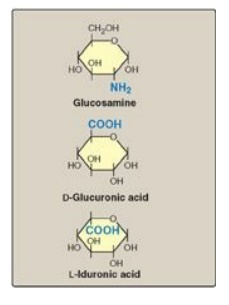
Figure 14.2 Some
monosaccharide units found in glycosaminoglycans.
A. Relationship between glycosaminoglycan structure
and function
Because of their large
number of negative charges, these heteropolysaccharide chains tend to be
extended in solution. They repel each other and are surrounded by a shell of
water molecules. When brought together, they “slide” past each other, much as
two magnets with the same polarity seem to slide past each other. This produces
the “slippery” consistency of mucous secretions and synovial fluid. When a
solution of GAGs is compressed, the water is “squeezed out,” and the GAGs are
forced to occupy a smaller volume. When the compression is released, the GAGs spring
back to their original, hydrated volume because of the repulsion of their
negative charges. This property contributes to the resilience of synovial fluid
and the vitreous humor of the eye (Figure 14.3).
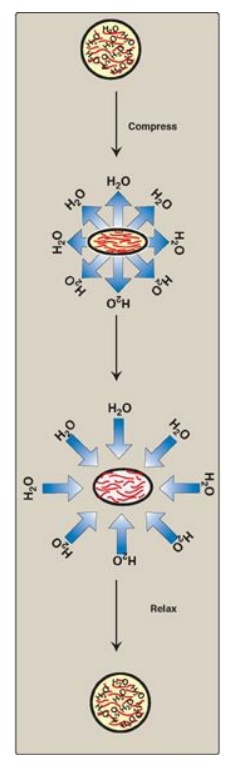
Figure 14.3 Resilience of glycosaminoglycans.
B. Classification of the glycosaminoglycans
The six major types of glycosaminoglycans are divided according to monomeric composition, type of glycosidic linkages, and degree and location of sulfate units. The structure of the GAGs and their distribution in the body is illustrated in Figure 14.4. All GAGs, except for hyaluronic acid, are sulfated and are found covalently attached to protein, forming proteoglycan monomers.
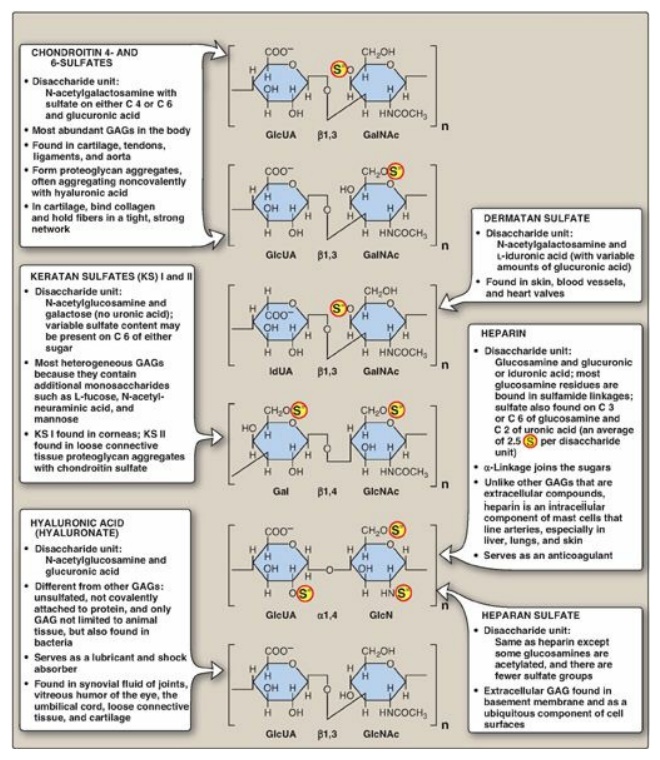
Figure 14.4 Structure and
distribution of glycosaminoglycans (GAGs). Sulfate groups ( S )
are shown in all possible positions. GlcUA = glucuronic acid; IdUA = iduronic
acid; GalNAc = N-acetylgalactosamine; GlcNAc = N-acetylglucosamine; GlcN =
glucosamine; Gal = galactose.
C. Proteoglycans
Proteoglycans are found
in the ECM and on the outer surface of cells.
1. Structure of proteoglycan monomers: A proteoglycan monomer found in
cartilage consists of a core protein to which up to 100 linear chains of GAGs
are covalently attached. These chains, which may each be composed of up to 200
disaccharide units, extend out from the core protein and remain separated from
each other because of charge repulsion. The resulting structure resembles a
“bottle brush” (Figure 14.5). In cartilage proteoglycan, the species of GAGs
include chondroitin sulfate and keratan sulfate. [Note: Proteoglycans are
grouped into gene families that encode core proteins with common structural
features. The aggrecan family (aggrecan, versecan, neurocan, and brevican),
abundant in cartilage, is an example.]
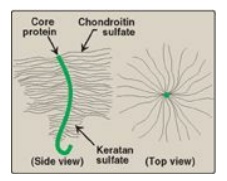
Figure 14.5 “Bottle-brush”
model of a cartilage proteoglycan monomer.
2. Linkage between the carbohydrate chain and the
protein: This
linkage is most commonly through a trihexoside (galactose-galactose-xylose) and
a serine residue, respectively. An O-glycosidic bond is formed between the
xylose and the hydroxyl group of the serine (Figure 14.6).
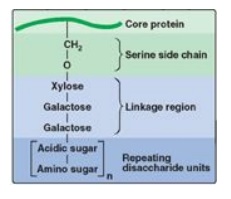
Figure 14.6 Linkage region of
glycosaminoglycans.
3. Proteoglycan aggregates: The proteoglycan monomers
associate with a molecule of hyaluronic acid to form proteoglycan aggregates.
The association is not covalent but occurs primarily through ionic interactions
between the core protein and the hyaluronic acid. The association is stabilized
by additional small proteins called link proteins (Figure 14.7).
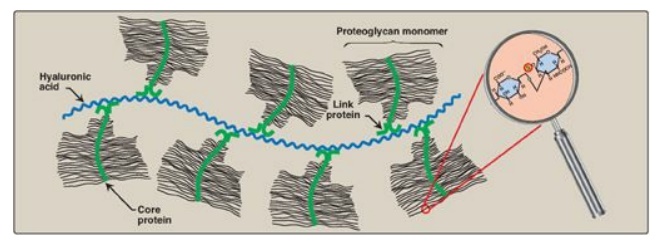
Figure 14.7 Proteoglycan aggregate.
Related Topics
