Overview of Glycoproteins
| Home | | Biochemistry |Chapter: Biochemistry : Glycosaminoglycans, Proteoglycans, and Glycoproteins
Glycoproteins are proteins to which oligosaccharides are covalently attached.
OVERVIEW OF GLYCOPROTEINS
Glycoproteins are
proteins to which oligosaccharides are covalently attached. They differ from
the proteoglycans in that the length of the carbohydrate chain in glycoproteins
is relatively short (usually two to ten sugar residues in length, although they
can be longer), whereas it can be very long in the GAGs of proteoglycans. In
addition, whereas GAGs have repeating disaccharide units, the carbohydrates of
glycoproteins do not have serial repeats. The glycoprotein carbohydrate chains
are often branched instead of linear and may or may not be negatively charged.
Glycoproteins contain highly variable amounts of carbohydrate but typically
less than that in GAGS. For example, immunoglobulin IgG contains less than 4%
of its mass as carbohydrate, whereas human gastric glycoprotein (mucin)
contains more than 80% carbohydrate. Membrane-bound glycoproteins participate
in a broad range of cellular phenomena, including cell-surface recognition (by
other cells, hormones, and viruses), cell-surface antigenicity (such as the
blood group antigens), and as components of the ECM and of the mucins of the
gastrointestinal and urogenital tracts, where they act as protective biologic
lubricants. In addition, almost all of the globular proteins present in human
plasma are glycoproteins, although albumin is an exception. (See Figure 14.13
for a summary of some of the functions of glycoproteins.) [Note: Glycosylation
is the most common posttranslational modification of proteins.]
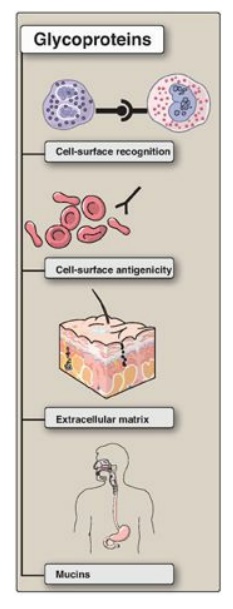
Figure 14.13 Functions of
glycoproteins.
Related Topics
