Chapter Summary, Questions Answers - Pentose Phosphate Pathway and Nicotinamide Adenine Dinucleotide Phosphate
| Home | | Biochemistry |Chapter: Biochemistry : Pentose Phosphate Pathway and Nicotinamide Adenine Dinucleotide Phosphate
The pentose phosphate pathway includes two irreversible oxidative reactions followed by a series of reversible sugar–phosphate interconversions .
CHAPTER SUMMARY
The pentose phosphate
pathway includes two irreversible oxidative reactions followed by a series of
reversible sugar–phosphate interconversions (Figure 13.14). No ATP is directly
consumed or produced in the cycle. The reduced nicotinamide adenine
dinucleotide phosphate (NADPH)-producing oxidative portion of the pentose
phosphate pathway is important in providing reducing equivalents for reductive
biosynthesis and detoxification reactions. In this part of the pathway, glucose
6-phosphate is irreversibly converted to ribulose 5-phosphate, and two NADPH
are produced. The regulated step is catalyzed by glucose 6-phosphate
dehydrogenase (G6PD), which is strongly inhibited by NADPH. Reversible
nonoxidative reactions interconvert sugars. This part of the pathway is the
source of ribose 5-phosphate, required for nucleotide and nucleic acid
synthesis. Because the reactions are reversible, they can be entered from
fructose 6-phosphate and glyceraldehyde 3-phosphate (glycolytic intermediates)
if ribose is needed and G6PD is inhibited. NADPH is a source of reducing
equivalents in reductive biosynthesis, such as the production of fatty acids in
liver, adipose tissue, and the mammary gland, and steroid hormones in the
placenta, ovaries, testes, and adrenal cortex. It is also required by red blood
cells (RBCs) for the reduction of hydrogen peroxide, providing the reducing
equivalents required by glutathione (GSH). GSH is used by glutathione
peroxidase to reduce peroxide to water. The oxidized glutathione (GSSH)
produced is reduced by glutathione reductase, using NADPH as the source of
electrons. NADPH provides reducing equivalents for the mitochondrial cytochrome
P450 monooxygenase system, which is used in steroid hormone synthesis in
steroidogenic tissue, bile acid synthesis in liver, and vitamin D activation in
the liver and kidney. The microsomal system uses NADPH to detoxify foreign
compounds (xenobiotics), such as drugs and a variety of pollutants. NADPH
provides the reducing equivalents for phagocytes in the process of eliminating
invading microorganisms. NADPH oxidase uses molecular oxygen and electrons from
NADPH to produce superoxide radicals, which, in turn, can be converted to
peroxide by superoxide dismutase. Myeloperoxidase catalyzes the formation of
bactericidal hypochlorous acid from peroxide and chloride ions. Rare genetic
defects in NADPH oxidase cause chronic granulomatous disease characterized by
severe, persistent, infections and formation of granulomas. NADPH is required
for the synthesis of nitric oxide (NO), an important free radical gas that
causes vasodilation by relaxing vascular smooth muscle, acts as a
neurotransmitter, prevents platelet aggregation, and helps mediate macrophage
bactericidal activity. NO is made from arginine and O2 by three
different NADPH-dependent NO synthases (NOS). The endothelial (eNOS), and
neuronal (nNOS) isozymes constantly produce very low levels of NO for
vasodilation and neurotransmission, respectively. The inducible isozyme ( iNOS)
produces large amounts of NO for defense against pathogens. G6PD deficiency
impairs the ability of the cell to form the NADPH that is essential for the
maintenance of the GSH pool.
The cells most affected
are the RBCs because they do not have additional sources of NADPH. G6PD
deficiency is an X-linked disease characterized by hemolytic anemia caused by
the production of free radicals and peroxides following administration of
oxidant drugs, ingestion of fava beans, or severe infections. The extent of the
anemia depends on the amount of residual enzyme. Class I variants, the most
severe (and least common), are associated with chronic nonspherocytic hemolytic
anemia. Babies with G6PD deficiency may experience neonatal jaundice.
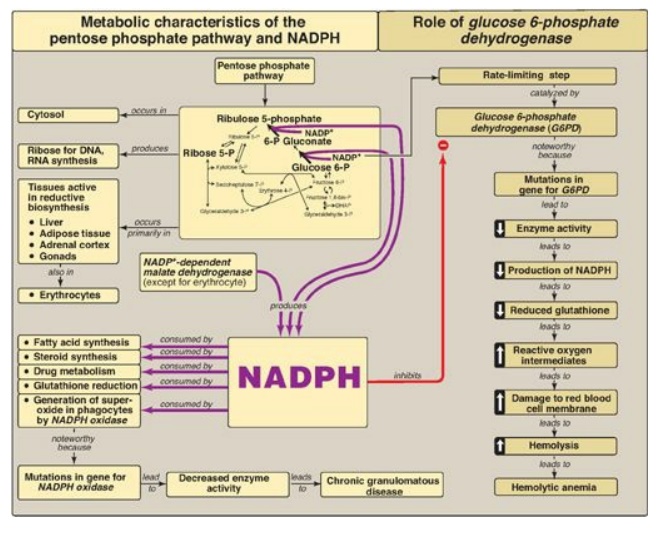
Figure 13.14 Key concept map for the pentose phosphate pathway and nicotinamide adenine dinucleotide phosphate (NADPH).
Study Questions
Choose the ONE best answer.
13.1 In preparation for
a trip to an area of India where chloroquine-resistant malaria is endemic, a
young man is given primaquine prophylactically. Soon thereafter, he develops a
hemolytic condition due to a deficiency in glucose 6-phosphate dehydrogenase. A
less-than-normal level of which of the following is a consequence of the enzyme
deficiency and the underlying cause of the hemolysis?
A. Glucose 6-phosphate
B. Oxidized form of
nicotinamide adenine dinucleotide
C. Reduced form of glutathione
D. Ribose 5-phosphate
Correct answer = C. Glutathione (GSH) is essential
for red cell integrity and is maintained in its reduced (functional) form by
nicotinamide adenine dinucleotide phosphate (NADPH)-dependent glutathione
reductase. The NADPH is generated by the oxidative portion of the pentose
phosphate pathway. Individuals with a deficiency of the initiating and
regulated enzyme of this pathway, glucose 6-phosphate dehydrogenase (G6PD),
have a decreased ability to generate NADPH and, therefore, a decreased ability
to keep GSH functional. When treated with an oxidant drug such as primaquine,
some patients with G6PD deficiency develop a hemolytic anemia. Primaquine does
not affect glucose 6-phosphate levels. Nicotinamide adenine dinucleotide is
neither produced by the pentose phosphate pathway nor used as a coenzyme by GSH
reductase.
13.2 Septic shock, a state of acute circulatory
failure characterized by persistent arterial hypotension (low blood pressure)
and inadequate organ perfusion refractory to fluid resuscitation, results from
a severe inflammatory response to bacterial infection. It has a high mortality
rate and is associated with changes in the level of nitric oxide. Which
statement concerning septic shock is most likely correct?
A. Activation of
endothelial nitric oxide synthase causes an increase in nitric oxide.
B. High mortality is
the result of the long half-life of nitric oxide.
C. Lysine, the nitrogen
source for nitric oxide synthesis, is deaminated by bacteria.
D. Overproduction of nitric oxide by a calcium-independent
enzyme is the cause of the hypotension.
Correct answer = D. Overproduction of short-lived (not
long-lived) nitric oxide (NO) by calcium-independent, inducible nitric oxide
synthase (iNOS) results in excessive vasodilation leading to hypotension. NOS
uses arginine, not lysine, as the source of the nitrogen. The endothelial
enzyme (eNOS) is constitutive and produces low levels of NO at a consistent
rate.
13.3 An individual who has recently been prescribed
a drug (atorvastatin) to lower cholesterol levels is advised to limit
consumption of grapefruit juice, because high intake of the juice reportedly
results in an increased level of the drug in the blood, increasing the risk of
side effects. Atorvastatin is a substrate for the cytochrome P450 enzyme
CYP3A4, and grapefruit juice inhibits the enzyme. Which statement concerning
P450 enzymes is most likely correct?
A. They accept
electrons from reduced nicotinamide adenine dinucleotide (NADH).
B. They catalyze the hydroxylation of hydrophobic
molecules.
C. They differ from
nitric oxide synthase in that they contain heme.
D. They function in
association with an oxidase.
Correct answer = B. The P450 enzymes hydroxylate
hydrophobic compounds, making them more water soluble. Reduced nicotinamide
adenine dinucleotide phosphate (NADPH) from the pentose phosphate pathway is
the electron donor. The electrons are first transferred to the coenzymes of
cytochrome P450 reductase and then to the P450 enzyme. Both the P450 enzymes
and the nitric oxide synthase enzymes contain heme.
13.4 In male patients who are hemizygous for
X-linked glucose 6-phosphate dehydrogenase deficiency, pathophysiologic
consequences are more apparent in red blood cells (RBC) than in other cells
such as in the liver. Which one of the following provides the most reasonable
explanation for this different response?
A. Excess glucose
6-phosphate in the liver, but not in RBC, can be channeled to glycogen, thereby
averting cellular damage.
B. Liver cells, in contrast to RBC, have
alternative mechanisms for supplying the reduced nicotinamide adenine
dinucleotide phosphate required for maintaining cell integrity.
C. Because RBC do not
have mitochondria, production of ATP required to maintain cell integrity
depends exclusively on the shunting of glucose 6-phosphate to the pentose
phosphate pathway.
D. In RBC, in contrast
to liver cells, glucose 6-phosphatase activity decreases the level of glucose
6-phosphate, resulting in cell damage.
Correct answer = B. Cellular damage is directly
related to decreased ability of the cell to regenerate reduced glutathione, for
which large amounts of reduced nicotinamide adenine dinucleotide phosphate
(NADPH) are needed, and red blood cells (RBCs) have no means other than the
pentose phosphate pathway of generating NADPH. It is decreased product (NADPH),
not increased substrate (glucose 6-phosphate), that is the problem. RBCs do not
have glucose 6-phosphatase. The pentose phosphate pathway does not generate
ATP.
13.5 An essential prosthetic group for several
enzymes of metabolism is derived from the vitamin thiamine. Measurement of the
activity of what enzyme in red blood cells could be used to determine thiamine
status in the body?
Red blood cells do not
have mitochondria and, so, do not contain mitochondrial thiamine pyrophosphate
(TPP)-requiring enzymes such as pyruvate dehydrogenase. However, they do
contain the cytosolic TPP-requiring transketolase, whose activity can be used
to assess thiamine status.
Related Topics
