SAR of Anilides
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Local Anaesthetics
General structure of anilides is represented as follows:
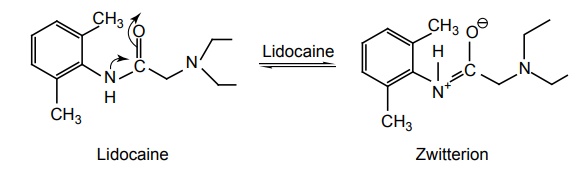
SAR of Anilides General structure of anilides is represented as follows: The clinically useful local anaesthetics of this type possess a phenyl group attached to the sp2 carbon atom through a nitrogen bridge. Placement of substituents on the phenyl ring with a methyl group in the 2 (or) 2 and 6-position enhances the activity. In addition, the methyl substituent provides steric hindrance to hydrolysis of the amide bond and enhances the coefficient of distribution. Any substitution on the aryl ring that enhances zwitterion formation will be more potent. ‘X’ may be carbon, oxygen, or nitrogen among them lidocaine series (X = O) has provided more useful products. The amino function has the capacity for salt formation and is considered as the hydrophilic portion of the molecule. Tertiary amines (diethyl amine, piperidine) are more useful because the primary and secondary amines are more irritating to tissues.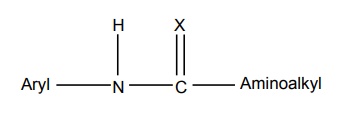
a. Aryl group
b. Substituent X
c. Amino alkyl group
