Sterilization Control and Sterility
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Sterilization Procedures And Sterility Assurance
To be labelled ‘sterile’, a product must be free of viable microorganisms. To achieve this, the product, or its ingredients, must undergo a sterilization process of sufficient microbiocidal capacity to ensure a minimum level of sterility assurance.
STERILIZATION CONTROL
AND STERILITY
To be labelled ‘sterile’, a product must be free of viable microorganisms. To achieve
this, the product,
or its ingredients, must undergo a sterilization
process of sufficient microbiocidal capacity
to ensure a minimum level of
sterility assurance. It is essential that the required
conditions for sterilization be achieved and maintained
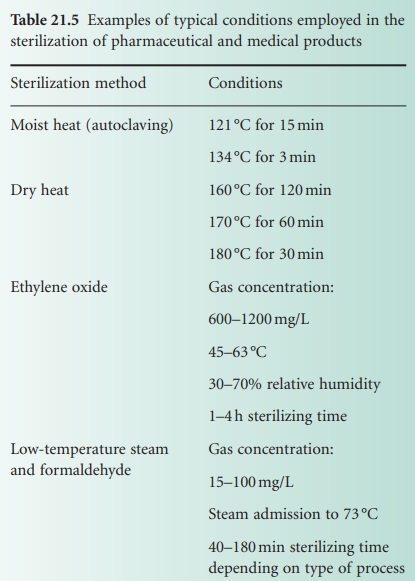
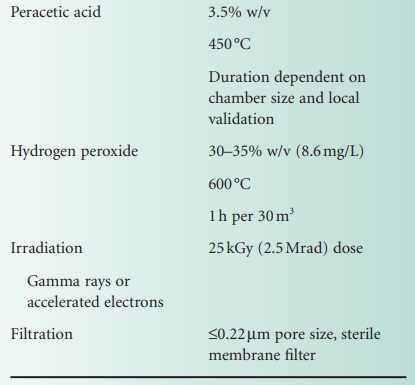
through every operation of the sterilizer. Some examples of typical conditions employed in sterilization are shown in Table 21.5.
Historically, the
quality control of sterile products consisted
largely, or in some
cases, even exclusively, of a
sterility test, to which the product was subjected at the
end of the manufacturing process.
However, a growing
awareness of the limitations of sterility tests
in terms of their ability to detect
low concentrations of microorganisms has resulted in a shift
in emphasis from a crucial dependence on end-testing to a situation in which the conferment of the status
‘sterile’ results from
the attainment of satisfactory quality
standards throughout the whole manufacturing process.
In other words, the quality is ‘assured’ by a combination of process monitoring and performance criteria; these may be considered under
four headings:
•
Bioburden determinations
•
Environmental monitoring
•
Validation and in-process monitoring of sterilization procedures
•
Sterility
testing.
In well-understood and well-characterized sterilization processes (e.g. heat and irradiation), where physical
measurements may be accurately made, sterility can be assured by ensuring that
the manufacturing process as a whole conforms to the established protocols for the first three of the above
headings. In this
case the process has satisfied the required parameters thereby permitting parametric release (i.e. release based on process
data) of the product without
recourse to a sterility test.
Related Topics
