Gaseous Sterilization - Sterilization Methods
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Sterilization Procedures And Sterility Assurance
The chemically reactive gases ethylene oxide [(CH 2) 2 O] and formaldehyde [(methanal, H.CHO)] possess broad-spectrum biocidal activity, and have found application in the sterilization of reusable surgical instruments, certain medical, diagnostic and electrical equipment, and the surface sterilization of powders.
GASEOUS STERILIZATION
The chemically reactive gases ethylene
oxide [(CH 2) 2 O] and formaldehyde [(methanal, H.CHO)]
possess broad-spectrum biocidal activity, and have found application in the
sterilization of reusable surgical instruments, certain medical, diagnostic and
electrical equipment, and the surface sterilization of powders. Sterilization
processes using ethylene oxide sterilization are far more commonly used on an
international basis than those employing formaldehyde.
Ethylene oxide treatment can also be considered as an alternative to
radiation sterilization in the commercial production of disposable medical
devices . These techniques do not, however, offer the same degree of sterility
assurance as heat methods and are generally reserved for temperature-sensitive
items.
The mechanism of antimicrobial action
of the two gases is assumed to be through alkylation of sulphydryl, amino,
hydroxyl and carboxyl groups on proteins and imino groups of nucleic acids. At
the concentrations employed in sterilization protocols, type A survivor curves (Figure 21.1)
are produced, the lethality of these gases increasing in a non-uniform manner
with increasing concentration, exposure temperature and humidity. For this
reason, sterilization protocols have generally been established by an empirical
approach using a standard product load containing suitable biological indicator
test strips (section 12.3). Concentration ranges (given as weight of gas per
unit chamber volume) are usually of the order of 800–1200 mg/L for ethylene
oxide and 15–100 mg/L for formaldehyde, with operating temperatures in the region
of 45–63 °C and 70–75 °C, respectively. Even at the higher concentrations and
temperatures, the sterilization processes are lengthy and therefore unsuitable
for the re sterilization of high-turnover articles. Further delays occur
because of the need to remove toxic residues of the gases before release of the
items for use. In addition, because recovery of survivors in sterility tests is
more protracted with gaseous sterilization methods than with other processes,
an extended quarantine period may also be required.
As alkylating agents, both gases are
potentially mutagenic and carcinogenic (as is the ethylene chlorohydrin that
results from ethylene oxide reaction with chlorine); they also produce symptoms
of acute toxicity including irritation of the skin, conjunctiva and nasal
mucosa. Consequently, strict control of their atmospheric concentrations is
necessary and safe working protocols are required to protect personnel. Table 21.3 summarizes
the comparative advantages afforded by ethylene oxide and low-temperature steam
and formaldehyde (LTSF) processes.
a) Ethylene Oxide
Ethylene oxide gas is highly explosive in mixtures of more than 3.6% v/v
in air; in order to reduce this explosion hazard it is usually supplied for
sterilization purposes as a 10% mix with carbon dioxide, or as an 8.6% mixture
with HFC 124 (2-chloro-1,1,1,2 tetrafluoroethane), which has replaced
fluorinated hydrocarbons (freons). Alternatively, pure ethylene oxide gas can
be used below atmospheric pressure in sterilizer chambers from which all air
has been removed.
The efficacy of ethylene oxide treatment depends on achieving a suitable
concentration in each article and this is assisted greatly by the good
penetrating powers of the gas, which diffuses readily into many packaging
materials including rubber, plastics, fabric and paper. This is not without its
drawbacks, however, as the level of ethylene oxide in a sterilizer will
decrease due to absorption during the process and the treated articles must
undergo a desorption stage to remove toxic residues. Desorption can be allowed
to occur naturally on open shelves, in which case complete desorption may take
many days, e.g. for materials like PVC, or it may be encouraged by special
forced-aeration cabinets where flowing, heated air assists gas removal,
reducing desorption times to between 2 and 24 hours.
Organisms are more resistant to ethylene oxide treatment in a dried
state, as are those protected from the gas by inclusion in crystalline or dried
organic deposits. Thus, a further condition to be satisfied in ethylene oxide
sterilization is attainment of a minimum level of moisture in the immediate
product environment. This requires a sterilizer humidity of 30–70% and
frequently a preconditioning of the load at relative humidities of more than
50%.
i) Sterilizer design and
operation
An ethylene oxide sterilizer consists of a leak-proof and explosion-proof
steel chamber, normally of 100–300 L capacity, which can be surrounded by a
hot-water jacket to provide a uniform chamber temperature. Successful operation
of the sterilizer requires removal of air from the chamber by evacuation,
humidification and conditioning of the load by passage of sub atmospheric-pressure
steam followed by a further evacuation period and the admission of preheated
vaporized ethylene oxide from external pressurized canisters or single-charge
cartridges. Forced gas circulation is often employed to minimize variations in
conditions throughout the sterilizer chamber. Packaging materials must be air,
steam-and gas-permeable to permit suitable conditions for sterilization to be
achieved within individual articles in the load. Absorption of ethylene oxide
by the load is compensated for by the introduction of excess gas at the beginning
or by the addition of more gas as the pressure drops during the sterilization
process. The same may also be true for moisture absorption, which is
compensated for by supplementary addition of water to maintain appropriate
relative humidity.
After treatment, the gases are
evacuated either directly to the outside atmosphere or through a special
exhaust system. Filtered, sterile air is then admitted either for a repeat of
the vacuum/air cycle or for air purging until the chamber is opened. In this
way, safe removal of the ethylene oxide is achieved, reducing the toxic hazard
to the operator. Sterilized articles are removed directly from the chamber and arranged
for desorption. The operation of an ethylene oxide sterilizer should be
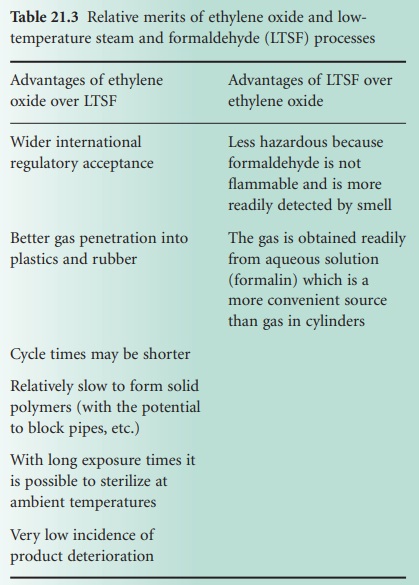
monitored and controlled automatically. A typical operating cycle for pure
ethylene oxide gas is shown in Figure 21.7.
b) Formaldehyde
Formaldehyde gas for use in sterilization is produced by heating formalin (37% w/v aqueous solution of formaldehyde) to a temperature of 70–75 °C with steam,
leading to the process
known as LTSF. Formaldehyde has a similar toxicity to ethylene oxide and although absorption
to materials appears
to be lower, similar
desorption routines are recommended. A major disadvantage of formaldehyde is low penetrating power, and this limits
the packaging materials that
can be employed to principally paper and cotton fabric.
i) Sterilizer design and operation
An LTSF sterilizer is designed to operate with
sub-atmospheric-pressure steam.
Air is removed by evacuation and steam
is admitted to the chamber
to allow heating of the load
and to assist
in air removal. The sterilization period starts
with the release
of formaldehyde by vaporization
from formalin (in a vaporizer with a steam jacket) and continues through
either a simple
holding stage or through a series of pulsed evacuations and steam and formaldehyde admission cycles. The chamber temperature is maintained by a thermostatically controlled water jacket,
and steam and condensate are removed
via a drain channel
and an evacuated condenser. At the end of the treatment period
formaldehyde vapour is expelled by steam
flushing and the load is dried by alternating stages
of evacuation and
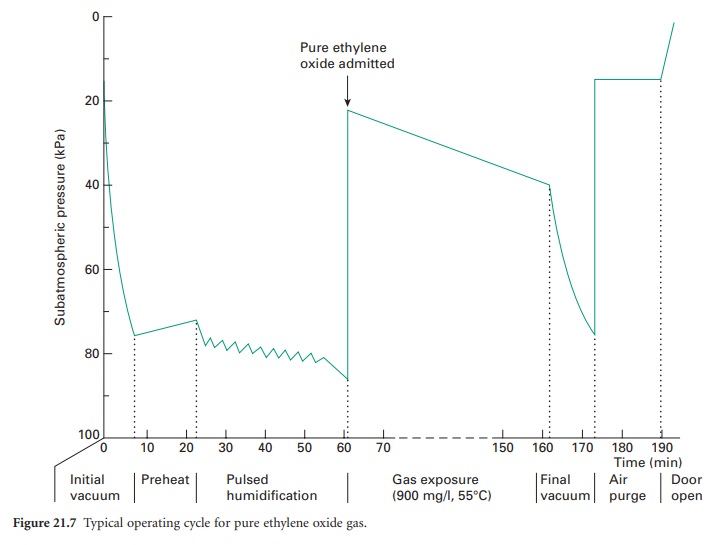
admission of sterile, filtered air. A typical
pulsed cycle of operation is shown in Figure 21.8.
c) Peroxygen Compounds
Hydrogen
peroxide (H2O2),
30–35%w/v, and peracetic acid (CH3CO3H), 3.5%w/v,
are used as highly effective
oxidizing agents
to kill microorganisms. The liquids
are heated to vaporize them and are held
in a sealed chamber where
all surfaces which
come into contact with the vapour
will be sterilized. These methods are widely
used for the sterilization of equipment used for
aseptic preparation and manufacture such
as isolators, with
the vaporized hydrogen
peroxide (VHP) often used for the routine
decontamination of cleanrooms.
Related Topics
