Thyroid Inhibitors
| Home | | Pharmacology |Chapter: Essential pharmacology : Thyroid Hormones And Thyroid Inhibitors
These are drugs used to lower the functional capacity of the hyperactive thyroid gland.
THYROID INHIBITORS
These
are drugs used to lower the functional capacity of the hyperactive thyroid
gland.
Thyrotoxicosis is due to excessive
secretion of thyroid hormones. The
two main causes are Graves’ disease and toxic nodular goiter. Graves’ disease is an autoimmune disorder: IgG
class of antibodies to the TSH receptor are detected in blood. They bind to and
stimulate thyroid cells, and produce other TSH like effects. Due to feedback
inhibition, TSH levels are low. The accompanying exophthalmos is due to
autoimmune inflammation of periorbital tissues.
Toxic
nodular goiter, which produces thyroid hormone independent of TSH, mostly supervenes
on old nontoxic goiters. It is more common in the elderly; ocular changes are generally
absent.
CLASSIFICATION
1.
Inhibit hormone synthesis
(Antithyroid drugs)
Propylthiouracil,
Methimazole, Carbimazole.
2.
Inhibit iodide trapping (Ionic
inhibitors)
Thiocyanates (–SCN),
Perchlorates (–ClO4), Nitrates (–NO3).
3.
Inhibit hormone release
Iodine, Iodides of Na
and K, Organic iodide.
4.
Destroy thyroid tissue
Radioactive iodine
(131I, 125I, 123I).
Compounds in groups 1
and 2 may be collectively called goitrogens.
In addition, certain
drugs used in high doses for prolonged periods cause hypothyroidism/goiter as a
side effect:
·
Lithium: inhibits thyroid hormone release.
·
Amiodarone: inhibits peripheral conversion of
T4 to T3; also interferes with thyroid hormone action.
·
Sulfonamides, paraaminosalicylic acid: inhibit
thyroglobulin iodination and coupling reaction.
·
Phenobarbitone, phenytoin, carbamazepine,
rifampin: induce metabolic degradation of T4/T3
Goitrin—found in
plants (cabbage, turnip, mustard, etc.), is the cause of goiter in cattle who
feed on these plants. May contribute to endemic goiter in certain iodine
deficient regions.
ANTITHYROID DRUGS
By convention, only
the synthesis inhibitors are called antithyroid drugs, though this term has
also been applied to all thyroid inhibitors.
Thiourea derivatives
were found to produce goiter and hypothyroidism in rats in the 1940s. Open
chain compounds were found to be toxic. Subsequently, methyl and propyl
thiouracil and thioimidazole derivatives methimazole and carbimazole were found
to be safe and effective.
Antithyroid drugs bind
to thyroid peroxidase and prevent oxidation of iodide/iodotyrosyl residues,
thereby;
i. Inhibit iodination of
tyrosine residues in thyroglobulin
ii. Inhibit coupling of
iodotyrosine residues to form T3 and T4.
Action (ii) has been
observed at lower concentration of antithyroid drugs than action (i). Thyroid
colloid is depleted over time and blood levels of T3/T4
are reduced.
They do not interfere
with trapping of iodide and do not modify the action of T3 and T4
on peripheral tissues or on pituitary. Goiter is not the result of potentiation
of TSH action on thyroid, but is due to increased TSH release as a consequence
of reduction in feedback inhibition. No goiter occurs if antithyroid drugs are
given to hypophysectomised animals or if T 4 is given along with
them. Antithyroid drugs do not affect release of T3 and T4—their
effects are not apparent till thyroid is depleted of its hormone content.
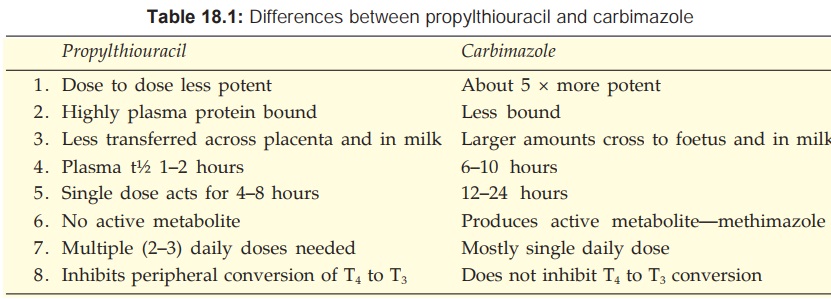
Propylthiouracil
also inhibits peripheral conversion of T4 to T3 by D1 type
of 5’DI, but not by D2 type. This may partly contribute to its effects. Methimazole
and carbimazole do not have this action (Table 18.1) and may even antagonize
that of propylthiouracil.
Pharmacokinetics
All
antithyroid drugs are quickly absorbed orally,
widely distributed in the body, enter milk and cross placenta; are metabolized
in liver and excreted in urine primarily as metabolites. All are concentrated
in thyroid: intrathyroid t½ is longer: effect of a single dose lasts longer
than would be expected from the plasma t½. Carbimazole acts largely by getting
converted to methimazole in the body.
Adverse Effects
Hypothyroidism and
goiter can occur due to
overtreatment, but is reversible on stopping the drug. It is indicated by
enlargement of thyroid, and is due to excess TSH production. Goiter does not
develop with appropriate doses which restore T4 concentration to
normal so that feedback TSH inhibition is maintained.
Important side effects
are: g.i. intolerance, skin rashes and joint pain.
Loss or graying of
hair, loss of taste, fever and liver damage are infrequent.
A rare but serious
adverse effect is agranulocytosis (1 in 500 to 1000 cases); It is mostly
reversible. There is partial cross reactivity between propylthiouracil and
carbimazole.
Preparations And Dose
Propylthiouracil: 50–150 mg TDS followed
by 25–50 mg BD–TDS for maintenance. PTU 50 mg tab.
Methimazole: 5–10 mg TDS initially, maintenance dose 5–15 mg daily in 1–2 divided doses.
Carbimazole: 5–15 mg TDS initially, maintenance dose 2.5–10 mg daily in 1–2 divided doses, NEO MERCAZOLE, THYROZOLE, ANTITHYROX
5 mg tab.
Carbimazole is more
commonly used in India. Propylthiouracil (600–900 mg/day) may be preferred in
thyroid storm for its inhibitory action on peripheral conversion of T4
to more active T3. It is also used in patients developing adverse
effects with carbimazole.
Use
Antithyroid drugs
control thyrotoxicosis in both Graves’
disease and toxic nodular goiter. Clinical improvement starts after 1–2 weeks
or more (depending on hormone content of thyroid gland). Iodide loaded patients
are less responsive. Maintenance doses are titrated on the basis of clinical
status of the patient. The following strategies are adopted.
As
definitive therapy (a) Remission may occur in upto half of the patients of Graves’
disease after 1–2 years of treatment: the drug can then be withdrawn. If
symptoms recur—treatment is reinstituted. This is preferred in young patients
with a short history of Graves’ disease and a small goiter.
Remissions are rare in toxic nodular goiter: surgery (or 131I)
is preferred. However, in frail elderly patient with multinodular goiter who
may be less responsive to 131I, permanent maintenance therapy with antithyroid
drugs can be employed.
Preoperatively Surgery in thyrotoxic patients is risky. Young patients with florid hyperthyroidism
and substantial goiter are rendered euthyroid with carbimazole before
performing subtotal thyroidectomy.
Along with 131I Initial control with antithyroid drug—1 to 2
weeks gap—radioiodine dosing—resume antithyroid drug after 5–7 days and
gradually withdraw over 3 months as the response to 131I develops. This
approach is preferred in older patients who are to be treated with 131I, but
require prompt control of severe hyperthyroidism. This will also prevent
initial hyperthyroidism following 131I due to release of
stored
T4.
Advantages
of antithyroid drugs over surgery/ 131I are:
·
No surgical risk, scar or chances of injury to
parathyroids or recurrent laryngeal nerve.
·
Hypothyroidism, if induced, is reversible.
·
Can be used even in children and young adults.
Disadvantages
are:
·
Prolonged (often life long) treatment is
needed because relapse rate is high.
·
Not practicable in uncooperative/unintelligent
patient.
·
Drug toxicity.
During pregnancy thyroidectomy
and 131I are contraindicated. With antithyroid drugs risk of foetal
hypothyroidism and goiter is there. However, low doses of propylthiouracil are
preferred: its greater protein binding allows less transfer to the foetus. For
the same reason it is to be preferred in the nursing mother. However, some
reports of safety of methimazole during pregnancy have appeared.
Propylthiouracil is
also used in thyroid storm.
IONIC INHIBITORS
Certain monovalent
anions inhibit iodide trapping by the thyroid probably because of similar
hydrated ionic size— T4/T3 cannot be synthesized.
Thiocyanate also inhibits iodination at high doses. Their relative inhibitory
potency is—
SCN
1: CLO4 10: NO3 1/30
They
are toxic and not used now.
Thiocyanates: can cause
liver, kidney, bone marrow and brain toxicity.
Perchlorates: produce
rashes, fever, aplastic anaemia, agranulocytosis.
Nitrates: are weak
drugs, can induce methemoglobinaemia and vascular effects.
IODINE AND IODIDES
Though iodine is a constituent
of thyroid hormones, it is the fastest acting thyroid inhibitor. It is reduced
in the intestines to iodide and the response to iodine or iodides is identical.
The gland, if enlarged, shrinks, becomes firm and less vascular. The thyroid
status starts returning to normal at a rate commensurate with complete stoppage
of hormone release from the gland. The gland itself involutes and colloid is
restored. With daily administration, peak effects are seen in 10–15 days, after
which ‘thyroid escape’ occurs and thyrotoxicosis may return with greater
vengeance. Worsening of hyperthyroidism especially occurs in multinodular
goiter.
All facets of thyroid
function seem to be affected, but the most important action is inhibition of
hormone release—‘thyroid constipation’. Endocytosis of colloid and proteolysis
of thyroglobulin comes to a halt. The mechanism of action is not clear. It
appears to be a direct action on thyroid cells, though attenuation of TSH and
cAMP induced thyroid stimulation has been demonstrated. Excess iodide inhibits
its own transport in thyroid cells and may alter the redox potential of cells,
thus interfering with iodination → reduced T3/T4 synthesis
(WolffChaikoff effect).
Preparations And Dose
Lugol’s solution (5%
iodine in 10% Pot. iodide
solution): LUGOL’S SOLUTION, COLLOID IODINE 10%: 5–10 drops/day. COLLOSOL 8
mg iodine/5 ml liq.
Iodide
(Sod./Pot.) 100–300 mg/day (therapeutic), 5–10 mg/ day (prophylactic) for
endemic goiter.
Uses
1. Preoperative preparation for thyroidectomy: generally given for 10
days just preceding surgery. The aim is to make the gland firm, less vascular
and easier to operate on. Though iodide itself will lower the thyroid status,
it cannot be relied upon to attain euthyroidism which is done by use of
carbimazole before starting iodide. Propranolol may be given additionally for
rapid control of symptoms.
2. Thyroid
storm Lugol’s iodine (6–10
drops) or iodine containing
radiocontrast media (iopanoic acid/ipodate) orally are used to stop any further
release of T3/T4 from the thyroid and to decrease T4
to T3 conversion.
3. Prophylaxis of endemic goiter It is generally used as “iodized
salt”.
4. Antiseptic As tincture iodine,
etc. see Ch. No. 65.
Adverse Effects
1. Acute
reaction It occurs in sensitive
individuals only—swelling of lips, eyelids, angioedema of larynx (may be
dangerous), fever, joint pain, petechial haemorrhages, thrombocytopenia, lymphadenopathy.
2. Chronic overdose (iodism) Inflammation of
mucous membranes, salivation, rhinorrhoea, sneezing, lacrimation, swelling of
eyelids, burning sensation in mouth, headache, rashes, g.i. symptoms, etc. The
symptoms regress on stopping iodide ingestion.
Longterm
use of high doses can cause hypothyroidism and goiter.
Iodide may cause
flaring of acne in adolescents. Given to pregnant or nursing mothers, it may be
responsible for foetal/infantile goiter and hypothyroidism.
RADIOACTIVE IODINE
The
stable isotope of iodine is 127I. Its radioactive isotopes of medicinal
importance are:
131 I: physical half-life
is 8 days—most commonly used in medicine.
123I: physical half-life
is 13 hours—only rarely used diagnostically.
125I: physical half-life is 60 days.
Their
chemical behaviour is similar to the stable isotope.
131I emits X-rays as well as β particles. The former
are useful in tracer studies, as they traverse the tissues and can be monitored
by a counter, while the latter are utilized for their destructive effect on
thyroid cells. 131I is concentrated by thyroid, incorporated in
colloid—emits radiation from within the follicles. The β particles penetrate
only 0.5–2 mm of tissue. The thyroid follicular cells are affected from within,
undergo pyknosis and necrosis followed by fibrosis when a sufficiently large
dose has been administered, without damage to neighbouring tissues. With
carefully selected doses, it is possible to achieve partial ablation of
thyroid.
It
is used as sodium salt of 131I dissolved in water and taken orally.
Diagnostic
25–100
μ curie is given;
counting or scanning is done at intervals. No damage to thyroid cells occurs at
this dose.
Therapeutic
The
most common indication is hyperthyroidism
due to Graves’ disease or toxic nodular goiter. The average therapeutic dose is 3–6 m
curie—calculated on the basis of previous tracer studies and thyroid size.
Higher doses are generally required for toxic multinodular goiter than for
Graves’ disease. The response is slow— starts after 2 weeks and gradually
increases, reaching peak at 3 months or so. Thyroid status is evaluated after 3
months, and a repeat dose, if needed, is given. About 20–40% patients require
one or more repeat doses.
Advantages
· Treatment with 131I is simple,
conveniently given on outpatient basis and inexpensive.
· No surgical risk, scar or injury to parathyroids/recurrent
laryngeal nerves.
· Once hyperthyroidism is controlled, cure is
permanent.
Disadvantages
· Hypothyroidism: About 5–10% patients of
Graves’ disease treated with 131I become hypothyroid every year
(upto 50% or more patients may ultimately require supplemental thyroxine
treatment). This probably reflects the natural history of Graves’ disease,
because only few patients of toxic nodular goiter treated with 131I
develop hypothyroidism. Moreover, eventual hypothyroidism is a complication of
subtotal thyroidectomy/prolonged carbimazole therapy as well.
·
Long latent period of response.
· Contraindicated during pregnancy—foetal
thyroid will also be destroyed resulting in cretinism, other abnormalities if
given during first trimester.
·
Not suitable for young patients: they are more
likely to develop hypothyroidism later and would then require lifelong T4
treatment. Genetic damage/cancer is also feared, though there is no evidence
for it.
131I is the treatment of choice after 25 years of
age and if CHF, angina or any other contraindication to surgery is present.
Metastatic carcinoma of thyroid (especially papillary or those cases of
follicular which concentrate iodine), 131I may be used as palliative
therapy after thyroidectomy. Much higher doses are required and prior
stimulation with TSH is recommended.
ADRENERGIC BLOCKERS
Propranolol (and other
nonselective β blockers) have
emerged as an important form of therapy to rapidly alleviate manifestations of
thyrotoxicosis that are due to sympathetic overactivity: palpitation, tremor,
nervousness, severe myopathy, sweating. They have little effect on thyroid
function and the hypermetabolic state. They are used in hyperthyroidism in the
following situations.
i. While awaiting response to carbimazole or 131I.
ii. Along with iodide for preoperative preparation
before subtotal thyroidectomy.
iii.
Thyroid storm (thyrotoxic crisis):
It is an emergency due to decompensated hyperthyroidism.
Vigorous treatment with the following is indicated:
• Nonselective β blockers are the most
valuable measure: afford dramatic symptomatic relief. In addition, they reduce
peripheral conversion of T4 to T3. Propranolol 1–2 mg
slow i.v. may be followed by 40–80 mg oral every 6 hours .
• Propylthiouracil 200–300 mg oral 6 hourly:
reduces hormone synthesis as well as peripheral T4 to T3
conversion.
• Iopanoic acid (0.5–1 g OD oral) or ipodate are
iodine containing radiocontrast media. They are potent inhibitors of thyroid
hormone release from thyroid, as well as of peripheral T4 to T3
conversion.
•
Corticosteroids (hydrocortisone 100 mg i.v. 8
hourly followed by oral prednisolone): help to tide over crisis, cover any
adrenal insufficiency and inhibit conversion of T4 to T3
in periphery.
• Diltiazem 60–120 mg BD oral may be added if tachycardia
is not controlled by propranolol alone.
• Rehydration, anxiolylics, external cooling and
appropriate antibiotics are the other measures.
Related Topics
