Zero-Order Half-Life
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Pharmacokinetics Basic Considerations
Half-life (t½) or half-time is defined as the time period required for the concentration of drug to decrease by one-half.
Zero-Order Half-Life
Half-life (t½) or half-time is defined as the time period required for the concentration of
drug to decrease by one-half. When t = t½, C = Co/2
and the equation 8.7 becomes:
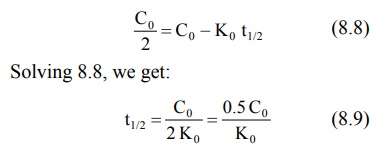
Equation 8.9 shows that the t½ of a
zero-order process is not constant but proportional to the initial
concentration of drug Co and inversely proportional to the
zero-order rate constant Ko. Since the zero-order t ½
changes with the decline in drug concentration, it is of little practical
importance. Zero-order equations do not require logarithmic transformations.
Examples of zero-order processes are –
1. Metabolism/protein-drug
binding/enzyme or carrier-mediated transport under saturated conditions. The rate
of metabolism, binding or transport of drug remains constant as long as its
concentration is in excess of saturating concentration.
2. Administration of a drug as a
constant rate i.v. infusion.
3. Controlled drug delivery such as that from i.m.
implants or osmotic pumps.
Related Topics