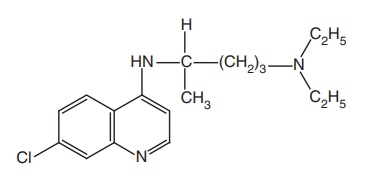
4-Substituted Quinolines
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antimalarials
4-Substituted Quinolines : Chloroquine (Nivaquin, Aralen, Lariago), Amodiaquine HCl (Camoquin), Hydroxy chloroquine (Plaquenil)
Antimalarials - Synthesis and Drug Profile
4-Substituted Quinolines
Mode of Action: Three different mechanism of actions are
suggested for these drugs:
DNA interaction: The mechanism of action for quinine is that
the drug gets intercalated into the DNA of the parasite. It is based on the
fact that the concentration required for the inhibition of nucleic acid
synthesis is significantly higher than that necessary for the inhibition of the
plasmodium parasite.
Ferriprotoporphyrin IX: The plasmodium parasite utilizes host
haemoglobin as a source of amino acid. On digestion of the haemoglobin, the
haem is released as ferriprotoporphyrins IX and it produces haemoly sis of the
erythrocyte parasites. Therefore, ferriprotoporphyrin that is released is
converted into nontoxic products and they, in turn, to haemozoites by the
polymerase enzyme. The steps involved in the conversion to haemozoites are
inhibited by the chloroquine.
Weak base hypothesis: The 4-substituted quinolines have weak base
and because of this pKa they are thought to accumulate in a location, which is
acidic (parasite lysozome pH 4.8–5.2). As the extracellular fluid of the
parasite is at pH 7.4, the weak base will move towards a more acidic pH of
lysosome. Once the acid–base reaction occurs, elevating the pH in the lysozome,
that in turn reduces the parasite’s ability to digest haemoglobin, thus
reducing the availability of amino acids.
Metabolism of Quinine: It is metabolized in the liver to 2-hydroxy
derivative followed by additional hydroxylation on the quinoline ring with the
2,3-dihydroxy derivative, as the major metabolite. This metabolite has low
activity and is rapidly excreted in urine.
Properties and uses: Quinine hydrochloride exists as fine, silky
needles, often in clusters, colourless, soluble in water and in alcohol. It is
used in the treatment of malaria.
Assay: Dissolve the sample in alcohol, add 0.01 M hydrochloric acid,
and titrate with 0.1 M sodium hydroxide. Determine the end point
potentiometrically.
Chloroquine (Nivaquin, Aralen, Lariago)
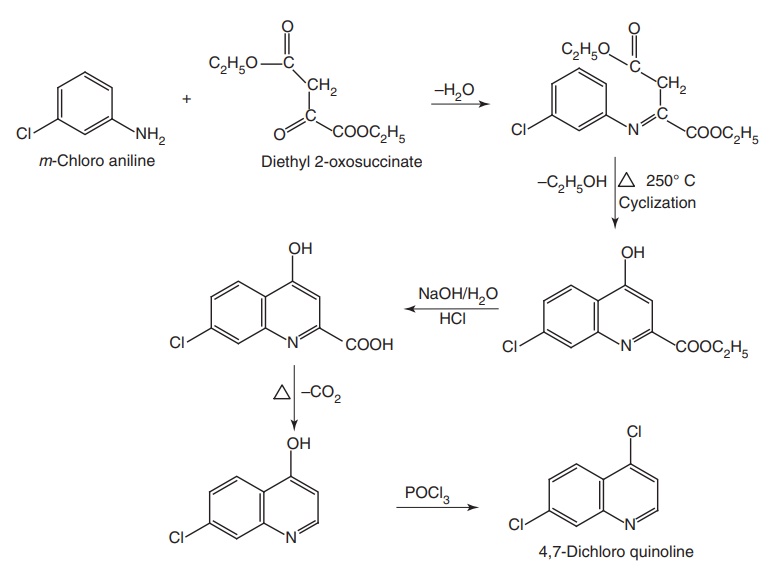
Synthesis
Step I. Synthesis of 4,7-dichloro quinolone
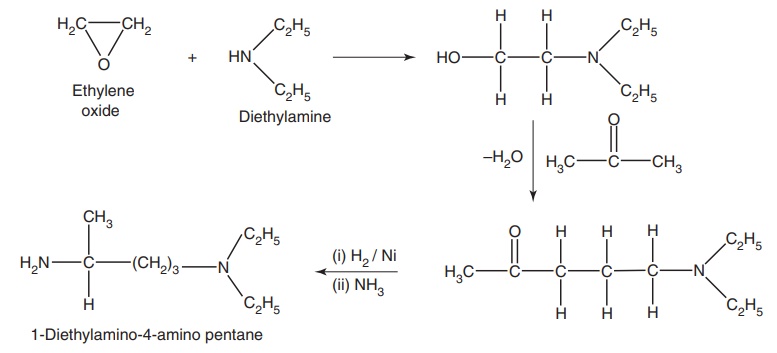
Step-II: Preparation of 1-diethyl amino-4-amino pentane
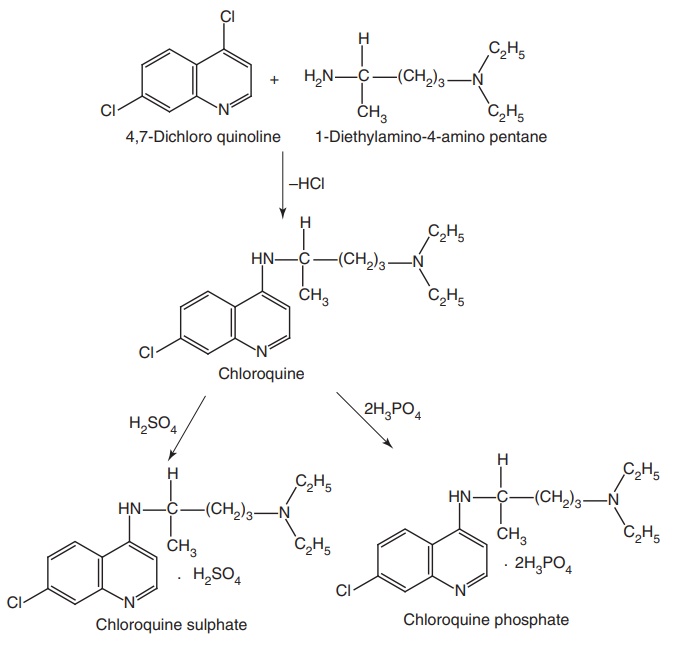
Step III. Condensation of the products of Step I and Step II
Metabolism: The drug is metabolized by N-dealkylation
through CYP2D6, and CYP3A4 isoenzymes. It has been reported that the level of
metabolism correlates closely with degree of resistance.
Properties and uses: Chloroquine exists as white or almost white
crystalline powder, soluble in water and in methanol, very slightly soluble in
ethanol. It is mainly used as an antimalarial. Chloroquine also has
antihistaminic and antiinflammatory properties. It is used to treat hepatic
amoebiasis, rheumatoid arthritis, discoid lupus erythematosus, cutanea tards,
solar urticaria, and polymorphous light eruptions. Chloroquine and other
4-amino quinolines are not effective against exoerythrocytic parasites. It is
an example for poor selective toxicity. Adverse reactions include retinopathy,
haemolysis in patients with glucose-6-phosphate dehydrogenase deficiency (same
mutation that confers resistance against malaria), muscular weakness,
exacerbation of psoriasis and porphyria, and impaired liver function.
Assay: Dissolve the sample in anhydrous acetic acid and titrate with
0.1 M perchloric acid. Determine the end point potentiometrically.
Dose: The recommended dose as a prophylactic and a suppressive is 500
mg once per week. As a therapeutic the dose , initially, is 1 g followed by 500
mg in 6 h, and 500 mg on the 2nd and 3rd day.
Dosage forms: Chloroquine sulphate injection I.P., B.P., Chloroquine sulphate
tablets I.P., B.P.
Amodiaquine HCl (Camoquin)
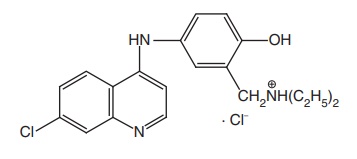
Synthesis
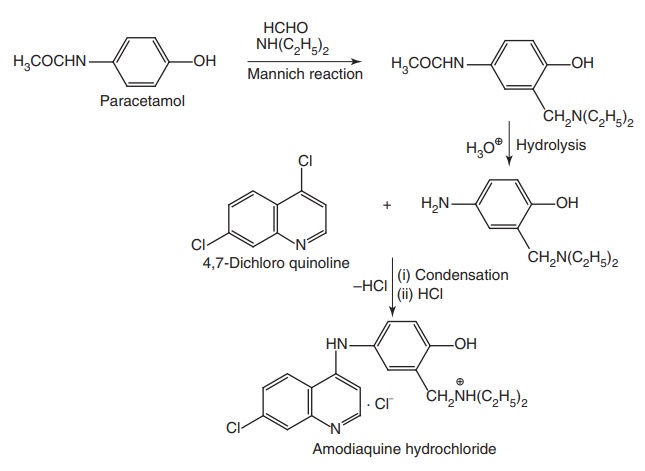
Properties and uses: It exists as yellow crystalline powder with a
bitter taste, and is soluble in water. It is very similar to chloroquine and
does not have any advantages over the other 4-amino quinoline drugs. It is used
for suppressing P. vivax and P. falciparum infections being 3–4 times
more active than quinine.
Dose: The recommended dose initially is 600 mg followed by 300 mg
doses 6, 24, and 48 h later.
Hydroxy chloroquine (Plaquenil)
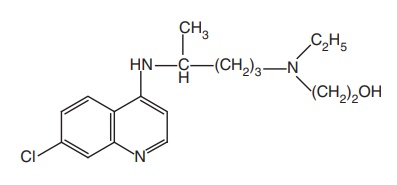
Synthesis
Step I. Preparation of side chain-N-ethyl-N-(2-hydroxyethyl)-4-amino pentylamine
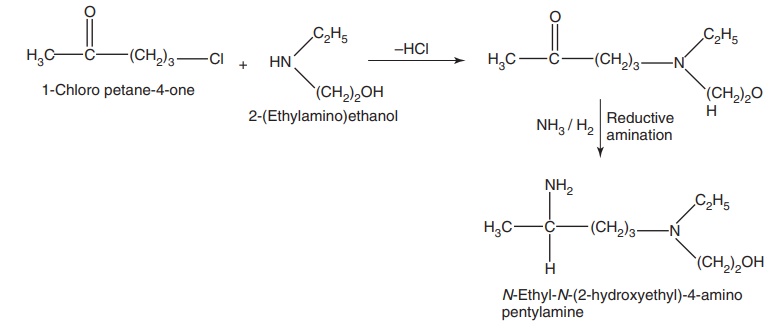
Step II. Condensation of the product of Step I with 4,7-dichloro quinolone
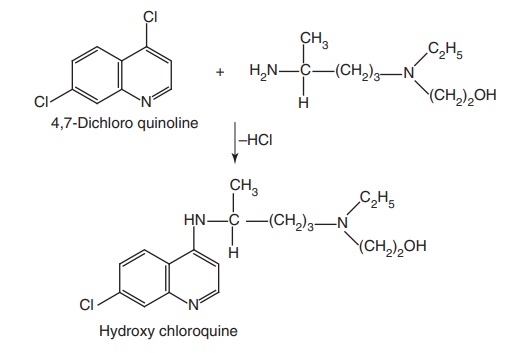
Properties and uses: Hydroxychloroquine is a white or almost white
crystalline powder, soluble in water,
practically insoluble in ethanol and in ether. It is equivalent to the
chloroquine, but it is less toxic and used in the place of chloroquine against
normally sensitive strains. It is mainly used as an antimalarial. It is also
used for the treatment of rheumatoid arthritis and lupus erythematoses.
Assay: Dissolve the sample in water, add 1 M sodium hydroxide, and
extract with dichloromethane. Combine the dichloromethane extracts and
evaporate. Add anhydrous acetic acid and titrate against 0.1 M perchloric acid,
using oracet blue B solution as indicator.
Dose: In P. falciparum infections,
the dose is 1.25 g in a single dose or in two divided doses at 6 h intervals;
in rheumatoid arthritis, 400 mg daily; in lupus erythematosus, 200 to 400 mg 1
or 2 times daily.
Dosage forms: Hydroxychloroquine tablets B.P.
Related Topics
