Acid-Base Balance
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Fluid, Electrolyte, and Acid Base Balance
1. Explain the difference between an acid and a base. 2. List the body’s major chemical buffer systems. 3. Describe two additional ways the body balances acids and bases.
Acid-Base
Balance
Electrolytes that dissociate in water to release hydro-gen
ions are called acids.
Electrolytes that release ions that combine with hydrogen ions are called bases. Homeostasis requires control of acid and base concentrations in body fluids. Most
hydrogen ions in body fluids begin as byproducts of metabolic processes. The
major sources of hydrogen ions are as follows:
■■ Aerobic
respiration of glucose: produces carbon
dioxide and water, forms carbonic acid, and releases hydrogen and
bicarbonate ions
■■ Anaerobic
respiration of glucose: produces lactic
acid, adding hydrogen ions to body fluids
■■ Incomplete
oxidation of fatty acids: produces acidic
ketone bodies to increase hydrogen ion concentration
■■ Oxidation
of sulfur-containing amino acids: yields
sulfuric acid, releasing hydrogen ions
■■ Hydrolysis
of phosphoproteins and nucleic acids: produces phosphoric acid, releasing
hydrogen ions
Strengths of Acids and Bases
Strong
acids dissociate to release hydrogen ions more completely, whereas weak
acids release them less com-pletely. An example of a strong acid is
hydrochloric acid and of a weak acid is carbonic acid. Bases release ions, such
as hydroxide ions, that combine with hydrogen ions, lowering their own
concentration. Examples of bases include sodium hydroxide and sodium
bicarbonate. Strong bases dissociate to release more hydroxide ions than weak
bases. Negative ions are often referred to as bases. They may combine with
strong acids; for example, bicarbonate ions may com-bine with hydrogen ions
from hydrochloric acid to form carbonic acid.
All functioning proteins, including enzymes, cytochromes,
and hemoglobin, are influenced by hydrogen concentrations. This is true because
of their abundant hydrogen bonds. Therefore, nearly all bio-chemical reactions
are influenced by fluid environ-ment pH, and there is close regulation of
acid-base balance. Variations in optimal pH are not excessive. Although the
normal pH of intracellular fluid is on average 7.0, in the arterial blood it is
7.4 and in the venous blood and interstitial fluid, 7.35. The lower pH in
venous blood and the cells is because of their larger amounts of carbon dioxide
and acidic metab-olites. Carbon dioxide combines with water to form carbonic
acid. The partial pressure of carbon dioxide (Pco2) is the most important
factor affecting the pH of body tissues.
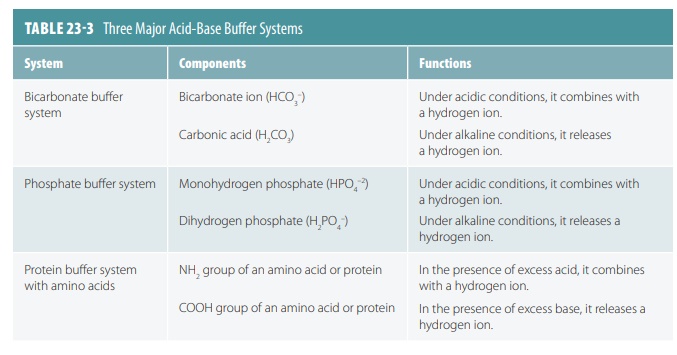
Acid-Base Buffer Systems
Acid-base
buffer systems consist of chemicals that combine with excess acids or bases. Buffer
sys-tem chemicals can combine with strong acids, which release more hydrogen
ions, to convert them into weak acids, which release fewer hydrogen ions. The
three most important acid-base buffer systems in the body’s fluids
are as follows:
■■ Bicarbonate buffer system: This system is present in both
intracellular and extracellular fluids, using the bicarbonate ion as a weak
base and carbonic acid as a weak acid. It is sometimes called the cabonic acid bicarbonate buffer system. Carbonic
acid is formed when hydrogen ions are excessive and dissociates when conditions
are basic or alkaline. The body maintains a readily available bicarbonate reserve.
■■ Phosphate buffer system: This system also operates in both
intracellular and extracellular fluids and is very important in controlling
hydrogen ion concentrations in the fluid of the nephrons and in urine. It consists
of monohydrogen phosphate and dihydrogen phosphate.
■■ Protein buffer system: Consists of plasma proteins and certain cell
proteins. When the solution pH falls, amino groups accept hydrogen ions; when
it rises, carboxyl groups release hydrogen ions. For red blood cells, which are
densely packed with hemoglobin molecules that buffer hydrogen ions, a chloride shift occurs. This involves
dissociation of carbonic acid and bicarbonate ions diffusing into the plasma in
exchange for chloride ions. In the lungs this occurs in reverse and is known as
the hemglobin buffer system. TABLE 23-3 summarizes
the three major buffer systems.
Carbonic acid production increases when cells increase
carbon dioxide production. As carbonic acid dissociates, hydrogen ions increase
and the inter-nal environment pH drops. These actions stimulate chemoreceptors
in the medulla oblongata, increas-ing breathing so the lungs can excrete more
carbon dioxide. If cells are less active, production of these components is
low and breathing may be closer to resting levels. Nephrons excrete hydrogen
ions in urine to help regulate hydrogen ion concentration. Epithelial cells in
the renal tubules secrete hydrogen ions into the tubular fluid.
1. Explain the difference between an acid and a base.
2. List the body’s major chemical buffer systems.
3. Describe two additional ways the body balances acids and
bases.
Related Topics