Acid-Base Imbalances
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Fluid, Electrolyte, and Acid Base Balance
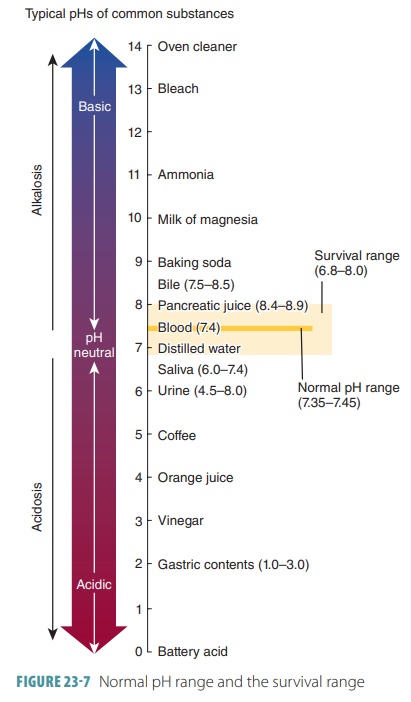
The pH of arterial blood is usually between 7.35 and 7.45. Abnormal values below 7.35 produce acidosis, whereas values above 7.45 produce alkalosis.
Acid-Base
Imbalances
The pH of arterial blood is usually between 7.35 and 7.45.
Abnormal values below 7.35 produce acidosis,
whereas values above 7.45 produce alkalosis.
Shifts in pH can be life threatening. Survival may be impos-sible if blood pH
is below 6.8 or above 8.0 for more than a few hours (FIGURE 23-7 ). The partial pressure of carbon
dioxide (Pco2) is the most important indica tor of normal
respiratory function. Normal levels are between 35 and 45 mm Hg. Dangerous
acidosis and alkalosis conditions may be linked to cardiovascu-lar,
respiratory, urinary, digestive, or nervous system abnormalities. To correctly
diagnose these conditions, most blood tests include screenings of pH and buffer
system function. Blood pH, Pco 2, and bicarbonate ion levels are
measured. Additional tests include measur-ing the anion gap and using nomograms
or diagnostic charts to plot test results. These steps help to correctly identify the condition, its severity,
its causes, and whether it is compensated or uncompensated.
Respiratory Acidosis
The two major types of acidosis are respiratory acido-sis and
metabolic acidosis. Respiratory acidosis may be caused by increased carbon dioxide concentration as well as
carbonic acid or respiratory acid and
may result in the following conditions:
■■ Injury to the brain stem’s
respiratory center, decreasing breathing
■■ Obstruction of air passages and
interference with air movement into alveoli
■■ Diseases decreasing gas exchange,
such as pneumonia, or reducing respiratory membrane surface area, such as
emphysema; also linked to cystic fibrosis
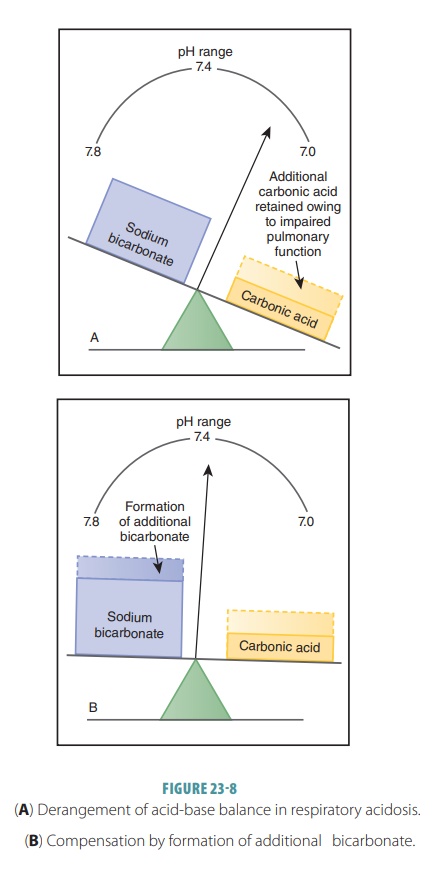
Respiratory acidosis is generally indicated by a Pco2
that is above 45 mm Hg, known as hyper-capnia,
and lowered blood pH. It is usually caused by hypoventilation, which is an abnormally low respiratory rate.
When the Pco2 rises in the extra-cellular fluid compartment,
hydrogen and bicar-bonate ion concentrations also rise. This occurs as carbonic
acid forms and dissociates. When buffer systems cannot keep up, the pH falls
rapidly. Just a few minutes of hypoventilation may result in acido-sis. The pH
of the extracellular fluid may reduce to as low as 7.0.
When the body’s chemical and physiological buffers return pH
to normal, the acidosis is compen-sated.
This is normally accomplished by chemorecep-tors that stimulate an increase in
breathing rate. In uncompensated acidosis,
the pH continues to drop, and the
patient can become comatose and eventu-ally die. Acute respiratory acidosis develops when the decline in pH is
severe. It is an especially dangerous condition when the patient’s tissues
generate large amounts of carbon dioxide or when normal respira-tory activity
is not possible. Therefore, for victims of cardiac arrest or drowning,
reversing acute respira-tory acidosis is the major goal. As a result, cardio-pulmonary
resuscitation, first aid, and lifesaving courses teach Airway, Breathing, and Circulation as the “ABCs” of emergency care.
Chronic
respiratory acidosis occurs because normal
respiratory function is compromised but compensatory mechanisms have not
completely failed. In a patient with central nervous system damage, normal
respiratory compensation may not occur even when stimulated by chemoreceptors.
People whose respiratory centers are desensitized by barbiturates or alcohol
may also be unable to achieve normal respiratory compensation. These
individuals often develop acidosis because of chronic hypoventilation. Other
factors, such as congestive heart failure, emphysema, pneumonia, pneumothorax,
and respiratory muscle paralysis, can influence the development of chronic
respira-tory acidosis.
When normal pulmonary responses are disabled, the kidneys
increase hydrogen ion secretion into the tubular fluid, slowing the rate of pH
change. Unfor-tunately, the kidneys are not able to return pH to nor-mal levels
on their own. The underlying circulatory or respiratory problems must be
corrected. Breath-ing efficiency may be temporarily improved with
bronchodilators or mechanical devices providing air that is under positive
pressure. Artificial respiration or mechanical ventilation are required once
breath-ing has ceased. If the respiratory acidosis was not severe or prolonged,
normal pH can still be restored. Respiratory acidosis treatment is made more
difficult because the condition also causes metabolic
acidosis as lactic acid is generated in tissues that do not have sufficient
oxygen. The effects of respiratory acidosis and the compensation for the
condition are shown in FIGURE
23-8.
Respiratory Alkalosis
Respiratory
alkalosis is a less common condition that results from excessive carbon dioxide and car-bonic acid
loss. Called hypocapnia, this
condition is signified by a Pco2 < 35 mm Hg, with raised blood
pH. A temporary hypocapnia can be produced by hyperventilation, often in response to anxiety, pain, fever, or
poisoning due to salicylates. Hyper-ventilation depletes carbon dioxide and
increases body fluid pH to as high as 8.0. Fortunately, respi-ratory alkalosis
is usually self-corrected, because chemoreceptor stimulation stops and the
urge to breathe reduces. Carbon dioxide levels then return to normal.
Hyperventilation often results from pain or other physical
stressors and extreme anxiety or other psychological stressors. It gradually
elevates the pH of the cerebrospinal fluid, affecting central nervous system
function. There are initial tingling sensations in the lips, hands, and feet.
The individual may be light-headed and lose consciousness if the condition
continues. Because unconsciousness stops percep-tion of causative psychological
stimuli, breathing rate declines and the condition is self-corrected. The
effects of respiratory alkalosis and compensation for the condition are shown
in FIGURE 23-9.
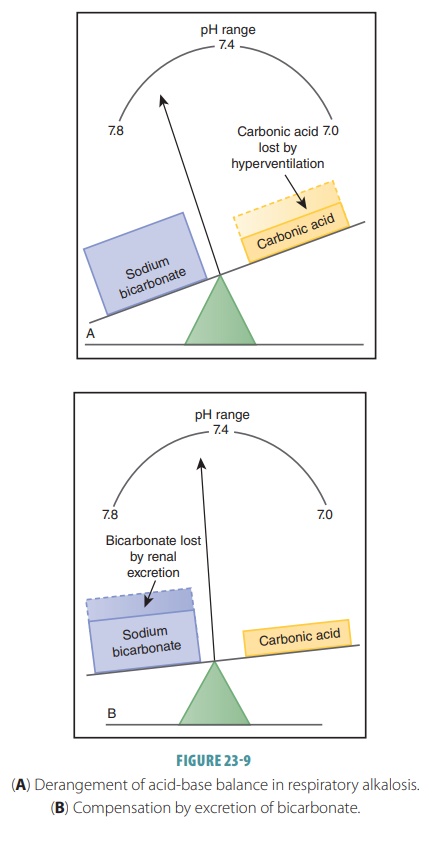
Hyperventilation is easily treated by having the patient rebreathe air that has been exhaled into a small paper bag. Rising Pco2 in the bag results in similar rises in the arterial and alveolar carbon dioxide concentrations. The pH is then restored to normal levels. Rare situations that may involve respiratory alkalosis include high altitudes that cause hyperventilation, use of mechanical respira-tors, and those with brain stem injuries that cause them to be unable to respond to changes in plasma carbon dioxide concentrations.
Metabolic Acidosis
Metabolic acidosis is the second most common type of
acid-base imbalance. Metabolic imbalances such as this are indicated by
bicarbonate levels below or above the normal range, which is 22–26 mEq/L.
Metabolic acidosis may be caused by accumulation of nonrespiratory acids or
loss of bases, such as in the following conditions:
■■ Lactic acidosis, which can develop
after strenuous exercise or prolonged tissue hypoxia, known as oxygen
starvation, as active cells rely on anaerobic respiration.
■■ Diabetes mellitus, which converts some
fatty acids into ketone bodies such as acetoacetic acid, beta-hydroxybutyric
acid, and acetone, causing ketonuria or ketoacidosis. This conversion of some fatty acids into ketone bodies
also occurs during starvation.
■■ Overconsumption of alcohol, which
is metabolized to acetic acid.
■■ Vomiting over a long period of time
causes the stomach to continue to generate stomach acids to replace those that
are lost. As a result, the bicarbonate concentration of the blood continues to
rise.
■■ Prolonged diarrhea, which is more
common in infants, causing excessive loss of bicarbonate ions.
■■ Kidney disease that reduces glomerular filtration and causes uremic acidosis; this is a less common condition. It may occur from glomerulonephritis and use of diuretics. When the reabsorption of sodium ions stops, secretion of hydrogen ions also stops.
Diagnosis and treatment of metabolic acidosis is based on
the cause. Although it can be easily linked to lactic acidosis after extreme
physical activity, there may be many more complicated causative factors. The
body generally compensates for metabolic acidosis via the lungs and kidneys.
The lungs eliminate carbon dioxide molecules formed by the interaction of
hydro-gen ions with bicarbonate ions. The kidneys excrete additional hydrogen
ions into the urine while gen-erating bicarbonate ions, which are released into
the extracellular fluid.
Metabolic and respiratory acidosis are often linked because
oxygen-starved tissues generate lactic acid in massive amounts and because
sus-tained hypoventilation results in decreased arterial partial pressure of
oxygen. Examples include near- drownings, in which there is high Pco2
and low par-tial pressure of oxygen in the body fluids. Lactic acid dominates
the muscles because of the attempts of the drowning person to stay above water.
Dissociation of lactic acids releases lactate and hydrogen ions. Emergency
treatment is vital, including artificial or mechanical respiratory assistance
and intravenous administration of an isotonic solution. This solution contains
sodium bicarbonate, sodium gluconate, or sodium lactate.
Metabolic Alkalosis
Metabolic
alkalosis results from excessive loss of hydrogen ions or gain of bases or bicarbonate ions. It is much less common than metabolic acidosis.
In metabolic alkalosis, there is in an increase in blood pH, called alkalemia,
after gastric drainage or lavage, use
of certain diuretics, overuse of antacids, or pro-longed vomiting. Loss of
acidic gastric juice leaves body fluids more basic. A condition called alkaline tide may occur, caused by many bicarbonate ions moving into the extracellular fluid. This movement is related to
secretion of hydrochloric acid from the gastric mucosa. Temporary elevation of
bicar-bonate ions in the extracellular fluid occurs during eating, but serious
metabolic alkalosis may occur because of repeated vomiting as the stomach
gen-erates more stomach acids to replace those regur-gitated. This means bicarbonate
ion concentrations in the extracellular fluid rise continually. Metabolic
alkalosis may also develop from taking excessive quantities of antacids.
Symptoms include decreased breathing rate and depth and
increased blood carbon dioxide. The com-pensatory factors for metabolic
alkalosis include reduced breathing rate, with a loss of bicarbonate ions in
the urine. For mild cases, treatment usually is focused on controlling vomiting
or treating other causative factors. Solutions that may be administered include
sodium chloride or potassium chloride. Acute metabolic alkalosis is treated
with ammonium chloride. As the ammonium ions are metabolized in the liver,
hydrogen ions are liberated, which basically means hydrochloric acid is
generated in greater quantities. As it diffuses into the bloodstream, the pH
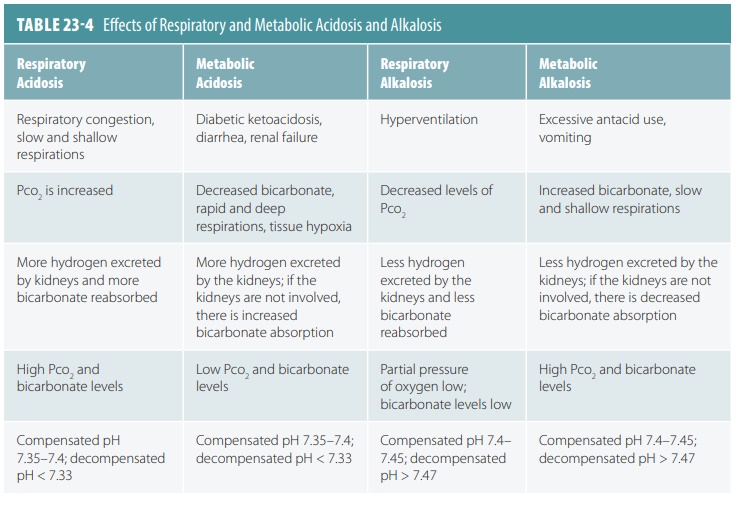
falls to normal levels. The effects of respiratory and metabolic acidosis and
alkalosis are described in TABLE
23-4.
Compensations for Imbalances
If the lung or kidney buffer systems become insuffi-cient,
the acid-base balance is disrupted. As a result, the undisturbed system tries
to compensate. The respiratory system is responsible for compensation of
metabolic acid-base imbalances and works rela-tively quickly. The urinary
system, although slower, is responsible for compensation of
respiratory-related acid-base imbalances. The ways these systems com-pensate
are reflected in changes in the Pco2 and concentrations of
bicarbonate ions. A patient can have a serious medical condition and still show
a normal pH because of how these systems compensate.
Respiratory Compensation
When the respiratory system compensates for a meta-bolic
acid-base imbalance, respiratory rate and depth change. They are usually elevated
in metabolic acido-sis. This is because high hydrogen ion levels stimulate the
respiratory centers. Blood pH is below 7.35, and bicarbonate ion levels are
below 22 mEq/L. The Pco2 falls below 35 mm Hg as carbon dioxide is
removed and excess acid leaves the blood. In respiratory aci-dosis, respiratory
rate is often depressed, which is the immediate cause of the acidosis. This is
not true for conditions of gas exchange impairment, such as pneumonia or
emphysema.
For metabolic alkalosis, respiratory compensa-tion involves
slow and shallow breathing. This allows carbon dioxide to accumulate in the
blood. Evidence of this compensation includes a pH above 7.45 at first, and
sometimes longer; bicarbonate levels over 26 mEq/L; and a Pco2 above
45 mm Hg.
Urinary Compensation
The kidneys speed up compensatory actions when an acid-base
imbalance is of respiratory cause. Acidosis is shown when a person is
hyperventilating. Although the kidneys are compensating, the levels of the Pco2,
as well as bicarbonate ions, are high. The raised Pco2 causes the
acidosis. The increasing bicarbonate ion level shows the kidneys are retaining
bicarbonate to compensate for the acidosis.
Oppositely, an individual who has respiratory alkalosis
compensated for by the kidneys has a high blood pH and a low Pco2.
As the kidneys eliminate more bicarbonate by not reclaiming it or by secreting
it, its levels begin to fall. However, the kidneys cannot compensate for either
alkalosis or acidosis if the con-dition is linked to a renal problem.
1. Explain the terms acidosis
and alkalosis.
2. What is “normal pH,” and what levels signify acidic or
alkaline pH levels?
3. Why can prolonged vomiting produce metabolic alkalosis?
4. What effect does a decrease in the pH of body fluids have
on the respiratory rate?