Antipseudomonal penicillins
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
Antipseudomonal penicillins: Carbenicillin
Penicillin - Synthesis and Drug Profile
Antipseudomonal penicillins
i. Carbenicillin
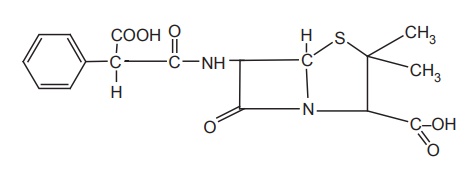
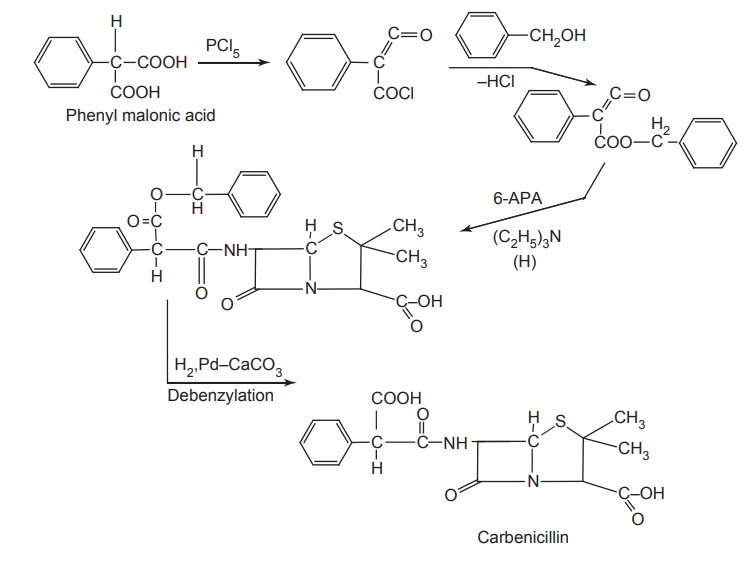
Synthesis
Properties and uses: It is a white to off white crystalline powder
with bitter taste, hygroscopic in nature, soluble in water or alcohol,
insoluble in chloroform or ether. It differs from ampicillin by having an
ionizable carboxyl group substituted on the alpha carbon atom of the benzyl
side chain rather than an amino group. The carboxyl group is thought to provide
improved penetration of the molecule through the cell wall barriers of
gram-negative bacilli as compared with other penicillins. A similar sequence
starting with 3-thiophenylmalonic acid leads to the ticarcillin. It is acid
labile being a malonic acid derivative, it decarboxylates readily to penicillin
G. It is effective in the treatment of systemic and urinary tract infections. It
has low toxicity, except allergic sensitivity, and the drug interferes with
platelet function resulting in bleeding.
Related Topics
