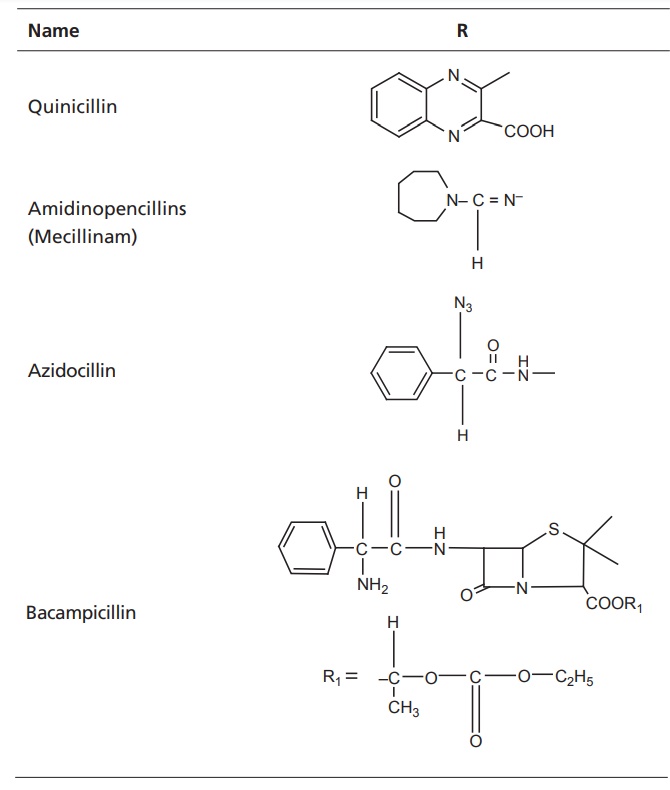
Classification of penicillins
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
I. Penicillinase-susceptible penicillins II. Penicillinase-resistant penicillins III. Aminopenicillins IV. Antipseudomonal penicillins (Carboxy Penicillins) V. Ureidopenicillins VI. Miscellaneous penicillins
CLASSIFICATION
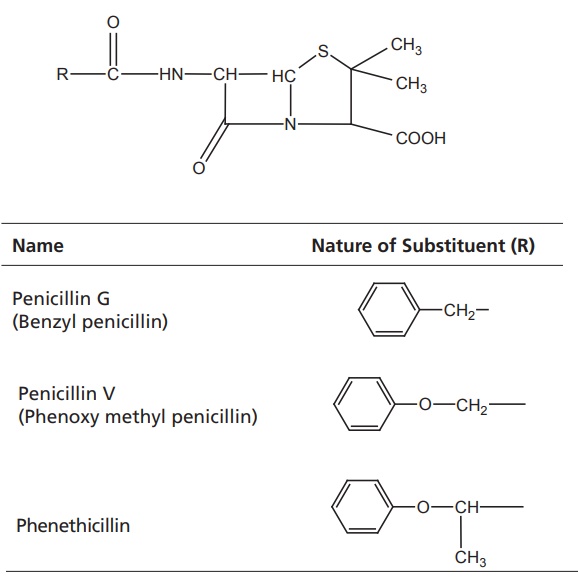
I. Penicillinase-susceptible
penicillins
The general
impact on antibacterial activity is as follows:
·Good gram-positive potency against susceptible Staphylococci and Streptococci
·Useful against some gram-positive cocci
·Not effective against gram-negative bacilli
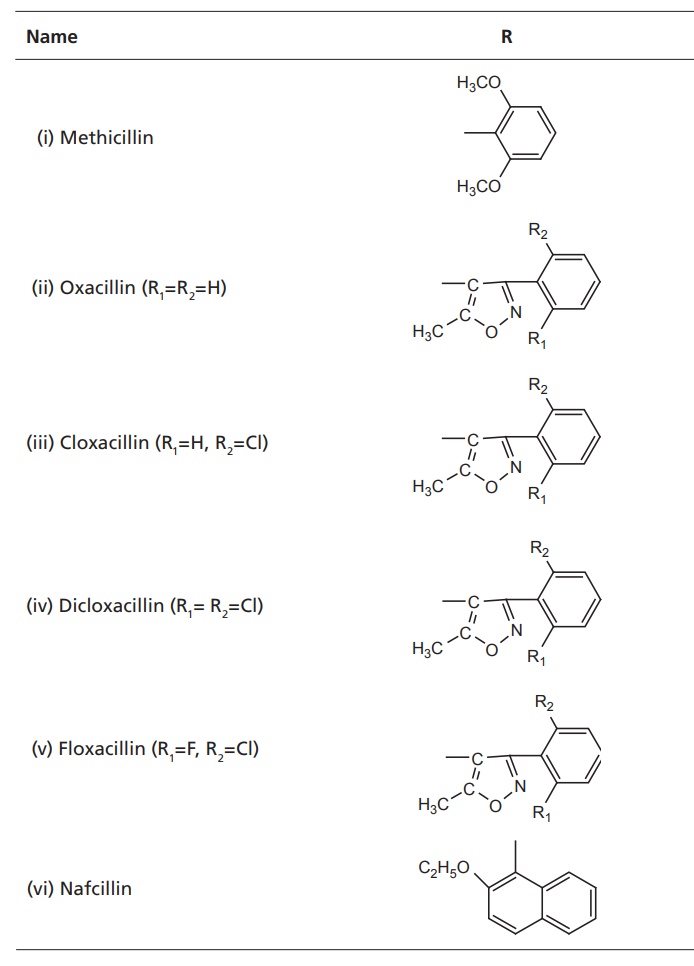
II. Penicillinase-resistant penicillins
General impact
on antibacterial activity is as follows:
·Decreased susceptibility to many penicillinase.
·Active against microrganisms, resistant to early
penicillin.
·Oxacillins offer good oral activity.
III. Aminopenicillins
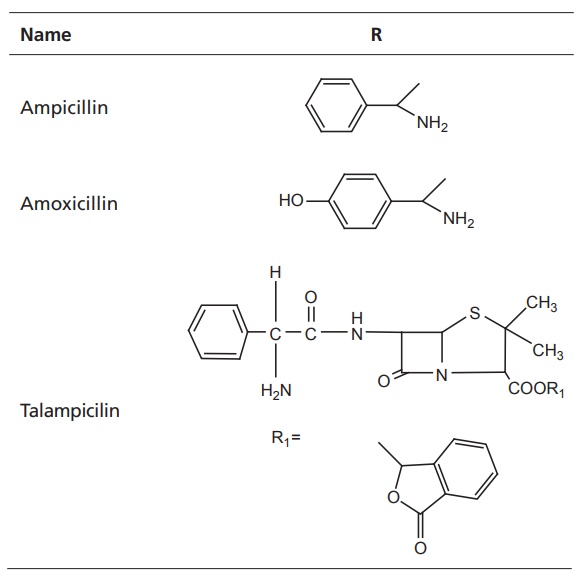
General
impact on antibacterial activity is as follows:
·Extended spectrum of activity against some
gram-negative bacteria and retention of gram-positive potency
·Ineffective against Pseudomonas aeruginosa
IV. Antipseudomonal penicillins (Carboxy Penicillins)

V. Ureidopenicillins
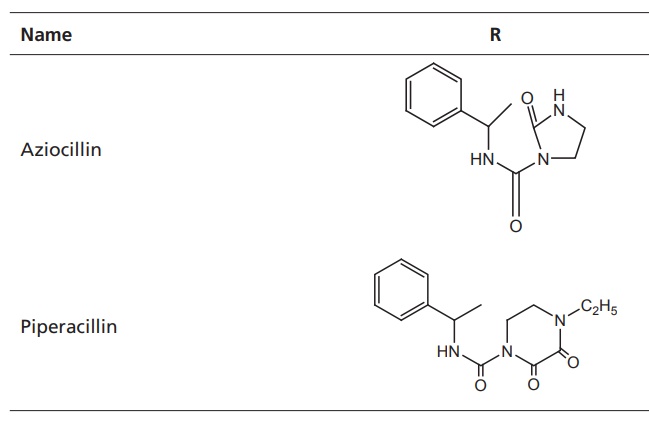
General
impact on antibacterial activity is as follows:
·Enhanced spectrum of activity against P. aeruginosa and expanded activity
against Klebsiella.
·Good potency against gram-positive bacteria, but
generally not effective against penicillinase producers.
·Good pharmacokinetic profile.
·Good activity against Escherichia coli, Klebsiella, Shigella, Salmonella, and many other
resistant species.
VI. Miscellaneous penicillins
The chemical
degradation of penicillins is depicted in Figure 4.1
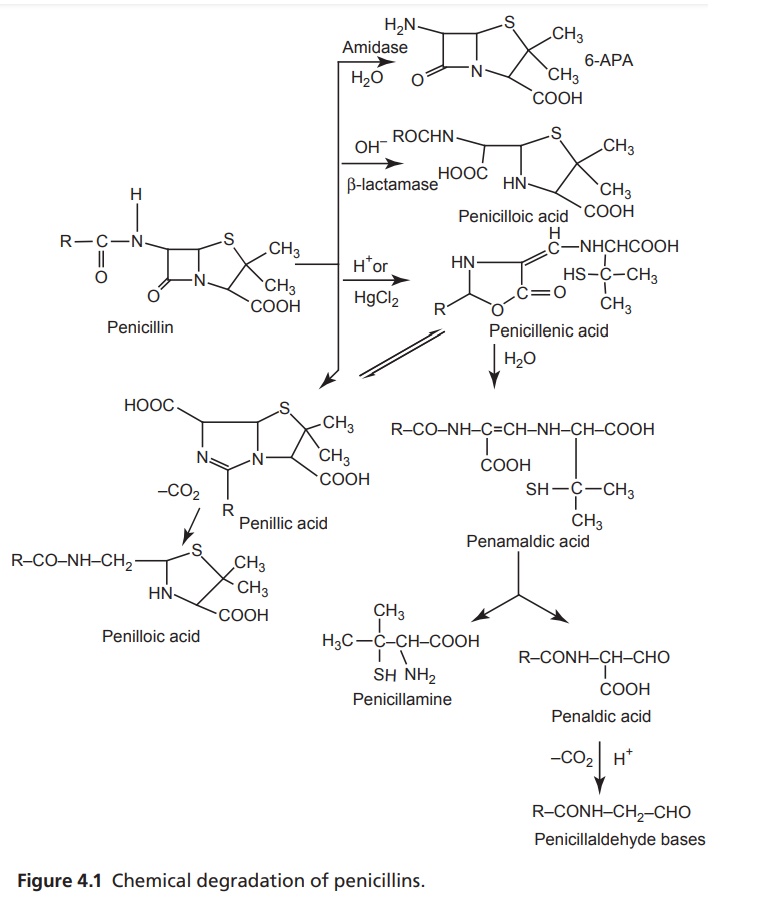
Inactivation
of penicillins by acids, bases, and β-lactamases is as follows:
·The penicillins are very reactive due to the
strained amide bond in the fused β-lactum of the nucleus.
·Penicillins undergo a complex series of
reactions leading to a variety of inactive degradation products.
·They are extremely susceptible to nucleophilic
attack by water or hydroxide ion to form the penicilloic acid. β-Lactamses also
cleave the β-lactam ring to give penicilloic acid with a consequent loss of
antibacterial activity.
·In strongly acidic solutions (pH < 3),
penicillin is protonated at the β-lactam nitrogen, and this is followed by
nucleophillic attack of the acyl oxygen atom on the β-lactam carbonyl carbon.
The subsequent opening of the β-lactam ring destabilizes the thiazoline ring,
which opens to form penicillenic acid that degrades into two major products
penicillamine and penilloic acid. A third product, penicilloaldehyde is also
formed.
·Acid-catalyzed degradation in the stomach
contributes in a major way to the poor oral absorption of penicillin. Thus,
efforts to obtain penicillins with improved pharmacokinetic and microbiologic
properties have sought to find acyl functionalities that would minimize
sensitivity of the β-lactam ring to acid hydrolysis and at the same time,
maintain antibacterial activity.
·Substitution of an electron-withdrawing group
for the α-position of the benzyl penicillin has stabilized the penicillin to
acid catalyzed hydrolysis. The increased stability imparted by such
electron-withdrawing groups has been attributed to a decrease in the reactivity
of the side chain amide carbonyl oxygen atom towards participation in β-lactam
ring opening to form the penicillenic acid.
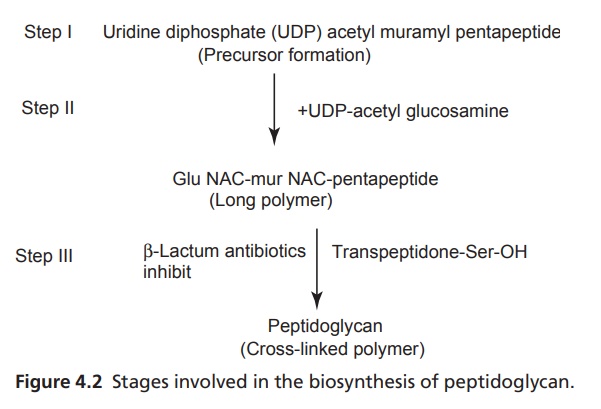
Mode of action: The cell wall of bacteria is essential for the
normal growth and development. Peptidoglycan is a heteropolymeric component of
the cell wall that provides rigid mechanism for stability by virtue of its
highly cross-linked lattice-wise structure. The peptidoglycan is composed of
glycan chains, which are linear strands of two alternating amino sugars (N-acetyl glucosamine and N-acetylmuramic acid) that are
cross-linked by peptide chains of an enzyme, transpeptidase. Penicillins inhibit
the transpeptidase activity to the synthesis of cell walls. They also block
cleavage of terminal D-alanine during the cell wall synthesis. The
biosynthesis of peptidoglycan involves three stages (Fig. 4.2).
B-lactam antibiotics inhibit the last step in
peptidoglycan synthesis. The transpeptidase enzyme that contains serine is
probably acylated by β-lactam antibiotics with the cleavage of -CO-N-bond of
the βlactam ring. This renders the enzyme inoperative and inhibits
peptidoglycan synthesis.
Related Topics
