SAR of Quinolones
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Quinolone Antibacterials
The optimum substituents at position 1 appear to be ethyl, butyl, cyclopropyl, and difluorophenyl, and these substituents have resulted in potent compounds.
SAR of Quinolones
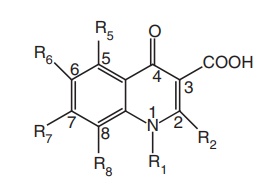
1. Substituent at N-1 position: The optimum substituents at position 1 appear to
be ethyl, butyl, cyclopropyl, and difluorophenyl, and these substituents have
resulted in potent compounds. Addition of a fluorine atom into the N-1
cyclopropyl group or the 1-butyl substituent resulted in compounds with overall
improved activity against gram-positive bacteria.
2. The
simple replacement of C-2 hydrogen has been generally disadvantageous (e.g. C-2
methyl or hydroxy groups); however, some derivatives containing a suitable C-1,
C-2 ring have shown to possess notable activity.
3. The carboxy functions at position: Modification of C-3 carboxylic acid group leads
to decrease in antibacterial activity. However, replacement of C-3 carboxylic
group with isothiazolo group afforded most active isothiazolo quinolone, which
has been 4–10 times greater in in vitro antibacterial
activity than ciprofloxacin. The isothiazolo system possesses aromatic character
and the nitrogen proton is very acidic and can be considered as an carboxylic
acid mimic, whereas other groups, such as sulphonic acid, phosphonic acid,
tetrazole as well as derivatization, as an ester lead to loss of antibacterial
activity.
4. The
C-4-oxo group of the quinolone nucleus appears to be essential for
antibacterial activity. Replacement with 4-thioxo or sulphonyl group leads to a
loss of activity.
5. The
incorporation of a group at the C-5 position has proven beneficial in terms of
antibacterial activity. The order of activity is NH2: CH3>F,
H>OH, or SH, SR.
6. The
incorporation of a fluorine atom at the C-6 position of the quinolone is
monumental. The order of activity is F>Cl, Br, CH3>CN.
7. The
introduction of a piperazine moiety at the C-7 position is essential. Other
aminopyrrolidines also are compatible for activity.
8. In
general, a C-8 fluoro substituent offers good potency against gram-negative
pathogens, while a C-8 methoxy moiety is active against gram-positive bacteria.
The order of activity is F, Cl, OCH3>H, CF3>methyl,
vinyl, propargyl.
9. A halogen (F or Cl) at the C-8 position improves oral absorption.
10. Linking
of N-1 group to the C-8 position with oxazine ring leads to active oflaxacin.
Uses: Fluoroquinolones are used to treat upper and lower respiratory
infections, gonorrhoea, bacterial gastroenteritis, skin soft tissue infections,
urinary tract infections, bone and joint infections, and against tuberculosis.
ADVERSE EFFECTS
The most
common adverse reactions are nausea, headache, and dizziness. Some CNS
problems, such as hallucination, insomnia, and visual disturbances can occur.
Some side effects of the quinolones are class effects and cannot be modulated
by molecular variation. Most of the fluoroquionolones produce photosensitivity
reactions and cause convulsions particularly in concurrent administration of
NSAID fenopofen. Increasing steric bulk through alkylation ameliorates these
effects. Phototoxicity is determined by the nature of the 8-position
substituent with halogen causing the greatest photoreaction while hydrogen and
methoxy show little light produced toxicity. These drugs are not recommended
for use in pretubertal children or pregnant women.
Related Topics
