Body Fluid Distribution
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Fluid, Electrolyte, and Acid Base Balance
Total body water changes with age, body mass, and relative amount of body fat. It is also different between the sexes.
Body
Fluid Distribution
Total body water changes with age, body mass, and relative
amount of body fat. It is also different between the sexes. Infants have
approximately 73% of their bodies made up of water, in part because they have
low body fat and low bone mass. Their skin is extremely soft because of this
high water content. Once the infant grows into childhood, the decline in total
body water has already begun. Healthy young boys have about 60% body water and
girls about 50%. By the time an individual is elderly, only about 45% of the
body mass consists of water. Gender differences in body water are related to
the general fact that females have more body fat and less skeletal muscle than
males. The least hydrated type of body tissue is adi-pose tissue, which
contains no more than 20% water. All other types of tissue, including bone,
have higher water contents. People with greater muscle mass have more body
water because skeletal muscle is made up of about 75% water.
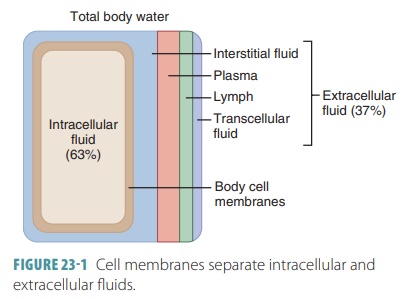
Fluid Compartments
Regions or compartments
of the body contain different volumes of fluids, with varying compositions.
Move-ment of water and electrolytes between compartments is regulated so they
are stable. The two major compart-ments are an intracellular fluid compartment
and an extracellular fluid compartment. The intracellular fluid compartment includes all water and
electro-lytes enclosed by cell membranes. In an adult, intra-cellular fluid
represents about 63%, by volume, of total body water. Therefore, the
intracellular fluid compart-ment in an adult man of approximately 150 pounds
accounts for about 25 of 40 total liters of body water.
The extracellular
fluid compartment includes all fluid outside of cells, making up about 37%,
by vol-ume, of total body water. This includes the plasma in the blood vessels,
the lymph in the lymphatic vessels, and the interstitial fluid in the tissue spaces. This
compartment is referred to as the body’s internal
envi-ronment. Some extracellular fluid is separated from other types of fluid and is known as transcellular fluid and includes
■■ Aqueous
and vitreous humors: in the eyes
■■ Cerebrospinal
fluid: in the central nervous system
■■ Secretions:
from the exocrine glands
■■ Serous
fluid: in body cavities
■■ Synovial
fluid: in the joints
FIGURE
23-1 shows how cell membranes separate intracellular and extracellular fluids
Fluid Composition
Many different types of solutes are dissolved in water, the universal solvent. Solutes are basically
classified as electrolytes or nonelectrolytes. Electrolytes include inorganic
salts, some proteins, acids, and bases. The acids and bases may be organic or
inorganic. Non-electrolytes have mostly covalent bonds, although other types of
bonds exist, which means they cannot dissociate in a solution. No electrically charged
par-ticles are created when they dissolve in water. Most nonelectrolytes are
organic molecules such as creati-nine, glucose, lipids, and urea.
Electrolytes have much more osmotic power than
nonelectrolytes, because their molecules dissociate into two or more ions. For
example, although the nonelec-trolyte glucose
remains undissociated and contributes one solute particle, a sodium chloride
(NaCl) molecule contributes two and a magnesium chloride (MgCl2)
contributes three. Both sodium chloride and magne-sium chloride are examples of
electrolytes. Sodium chloride dissociates into a sodium particle and a
chlo-ride particle. Magnesium chloride dissociates into a magnesium particle
and two chloride particles.
Water always moves according to osmotic gra-dients, regardless of the type of solute particles
con-tained, meaning water always moves from an area of lesser osmolality to an area of greater osmolality. As a result, electrolytes
have more ability to cause fluid shifts than nonelectrolytes. In the body fluids,
elec-trolyte concentrations are commonly expressed in milliequivalents per liter (mEq/L).
This measures the
number of electrical charges in 1 liter of solution. Any ion’s concentration
can be calculated in solution by using the following equation:
MEq/L = Concentration of ions (mg/L) / The ion’s atomic weight
(mg/mmol)
X Number of electrical charges on one ion
Notice in the equation the concentration of ions is
calculated in milligrams per liter. Also, the ion’s atomic weight is calculated
in milligrams per millimole. One
millimole is one -thousandth of a mole.
One mole is the base unit of amount of matter, which means a substance’s amount
that contains as many elementary entities as there are carbon atoms in 0.012
kg of carbon 12.
Therefore, to understand how this works, using sodium and
calcium as examples, we need to calcu-late the mEq/L for each. We determine the
normal concentrations of these ions in the plasma. Then we find their atomic
weights by using the periodic table. By using the equation, we find
the following for each:
Sodium = [3,300 mg/L] / [23 mg/mmol] × 1 particle = 143 mEq/L
Calcium = [100 mg/L] / [40 mg/mmol] × 2 particle = 5 mEq/L
Differences Between Extracellular and Intracellular Fluids
Most extracellular fluids contain high amounts of sodium and
chloride ions. They have lower amounts of magnesium, phosphate, and potassium
ions than do intracellular fluids. Blood plasma has much higher levels of
protein than interstitial fluid or lymph (FIGURE 23-2). In extracellular fluids, the primary cation is sodium and the primary
anion is chloride. Plasma
contains fewer chloride ions than the inter-stitial fluid because plasma is
electrically neutral, and nonpenetrating plasma proteins are usually anions.
The way these ions are distributed on either side of the cellular membranes
reflect the ATP-influenced activ-ity of the cellular sodium-potassium pumps.
These pumps keep intracellular sodium ion concentrations low, and potassium ion
concentrations high. The kid-neys assist by secreting potassium into the
filtrate, whereas sodium is reabsorbed from the filtrate.
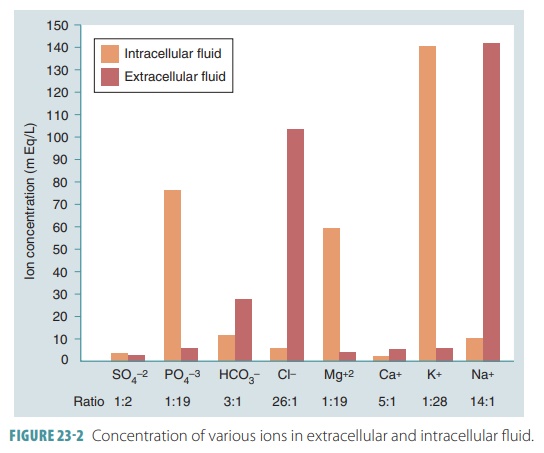
Intracellular fluid differs in that it has high amounts of
potassium, phosphate, and magnesium ions and low amounts of sodium and
chloride; it is basically opposite in its ion content to extracellular fluid.
In intracellular fluids, the primary cation is potassium and the primary anion
is hydrogen phos-phate. The cells additionally contain large quantities of
soluble proteins, in amounts that are about triple to those found in plasma.
Electrolytes are the most abundant solutes in the fluids of the body and control most chemical and physical reactions. However, they do not make up most dissolved solutes in the fluids. In the extracellu-lar fluid, proteins and certain nonelectrolytes such as cholesterol, phospholipids, and triglycerides are large molecules that are present. In the plasma, these make up approximately 90% of the mass of dissolved solutes and 60% in the interstitial fluid. In the intracellular fluid, they make up 97%.
Fluid Movement Between Compartments
Hydrostatic pressure and osmotic pressure regulate the
movement of water and electrolytes from one fluid compartment to another (FIGURE 23-3). Hydro-static pressure inside
cells and surrounding intersti-tial fluid is normally equal and stable.
Therefore, a change in osmotic pressure usually causes net fluid movement. The
net inward force is known as colloid osmotic pressure. When the levels of
sodium in the extracellular fluid decrease, this causes movement of water from
the extracellular compartment into the intracellular compartment, via osmosis.
Cells swell as a result. The opposite is true when sodium ion con-centration in
interstitial fluid increases, causing the cells to shrink.
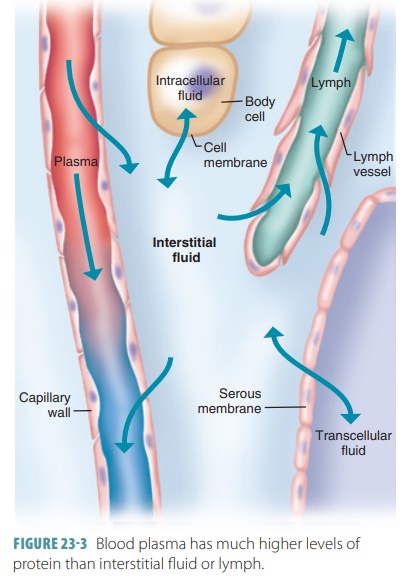
Although water moves freely between the com-partments, solutes are not equally distributed. This is due to their electrical charges, sizes, or need to use transport proteins. Basically, substances must pass through the plasma and interstitial fluid to reach the intracellular fluid. Exchanges between the plasma and “outside” environment are nearly continuous in the gastrointestinal tract, kidneys, and lungs. Plasma composition and volume are both altered. The plasma is the medium that allows substances to be delivered to all areas of the body. Balance is quickly restored by the body’s adjustments between the plasma, extracel-lular fluid, and intracellular fluid.
There are two key points: Exchanges between plasma and
interstitial fluid occur across capillary walls, and exchanges between the
interstitial fluid and intracellular fluid occur across plasma membranes. For
exchanges between plasma and interstitial fluid, the blood’s hydrostatic
pressure forces plasma that almost totally lacks proteins into the interstitial
space. The highly filtered fluid then is almost totally reabsorbed into the
bloodstream because of the colloid osmotic pressure of the plasma proteins.
Normally, lymphatic vessels pick up small amounts of net leakage remaining
behind in the interstitial space, returning it to the blood.
Exchanges across the plasma membranes are based on permeability. Generally, there is substantial two-way osmotic flow of water. Restriction of ion changes is based on ions moving selectively through channels or by active transport. Nutrients, respiratory gases, and wastes usually move in one direction. An example is how metabolic wastes move out of cells, whereas glu-cose and oxygen move into them. Except during the first minutes after a change in one type of body fluid, osmolalities of all body fluids are equal. When large amounts of pure water are consumed, the osmolalities of the two compartments are slightly lower.
1. Describe the percentages of extracellular fluid and
intracellular fluid in the body.
2. Explain which cations and anions are the primary ones found
in the extracellular fluid and in the intracellular fluid.
3. Describe the electrolytes that normally exist in the
extracellular fluid.
