Calcium
| Home | | Pharmacology |Chapter: Essential pharmacology : Drugs Affecting Calcium Balance
After C, O, H and N, calcium is the most abundant body constituent, making up about 2% of body weight: 1–1.5 kg in an adult. Over 99% of this is stored in bones, the rest being distributed in plasma and all tissues and cells. Calcium serves important physiological roles.
CALCIUM
After C, O, H and N, calcium is the most abundant body
constituent, making up about 2% of body weight: 1–1.5 kg in an adult. Over 99%
of this is stored in bones, the rest being distributed in plasma and all
tissues and cells. Calcium serves important physiological roles.
Physiological Roles
1. Calcium controls
excitability of nerves and muscles and regulates permeability of cell membranes.
It also maintains integrity of cell membranes and regulates cell adhesion.
2. Ca2+ ions are
essential for excitation-contraction coupling in all types of muscle and
excitation-secretion coupling in exocrine and endocrine glands, release of
transmitters from nerve ending and other release reactions.
3. Intracellular
messenger for hormones, autacoids and transmitters.
4. Impulse generation in
heart—determines level of automaticity and AV conduction.
5. Coagulation of blood.
6. Structural function in
bone and teeth.
Plasma Calcium Level
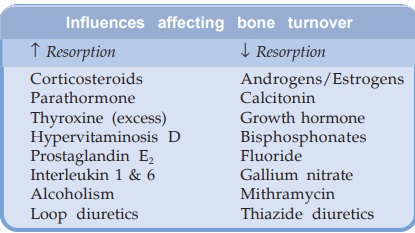
It is precisely
regulated by 3 hormones almost
exclusively devoted to this function, viz. parathormone
(PTH), calcitonin and calcitriol (active form of vit D). These
regulators control its intestinal
absorption, exchange with bone and renal excretion as summarized in Fig. 24.1.
In addition, several other hormones, metabolites and drugs influence calcium
homeostasis (see box).
Normal plasma calcium
is 9–11 mg/dl. Of this about 40% is bound to plasma proteins—chiefly albumin;
10% is complexed with citrate, phosphate and carbonate in an un-dissociable
form; the remaining (about 50%) is ionized and physiologically important. For
example, in hypoalbuminemia, total plasma calcium may be low but the concentration
of Ca2+ ion is usually normal. Acidosis favours and alkalosis disfavours ionization
of calcium: hyperventilation precipitates tetany and laryngospasm in calcium deficiency
by reducing ionization.
Calcium Turnover
Major fraction of
calcium in the bone is stored as
crystalline hydroxyapatite deposited on the organic bone matrix osteoid, while a small labile pool is in
dynamic equilibrium with plasma. Even the fully laid down parts of the bone
undergo constant remodeling by way of
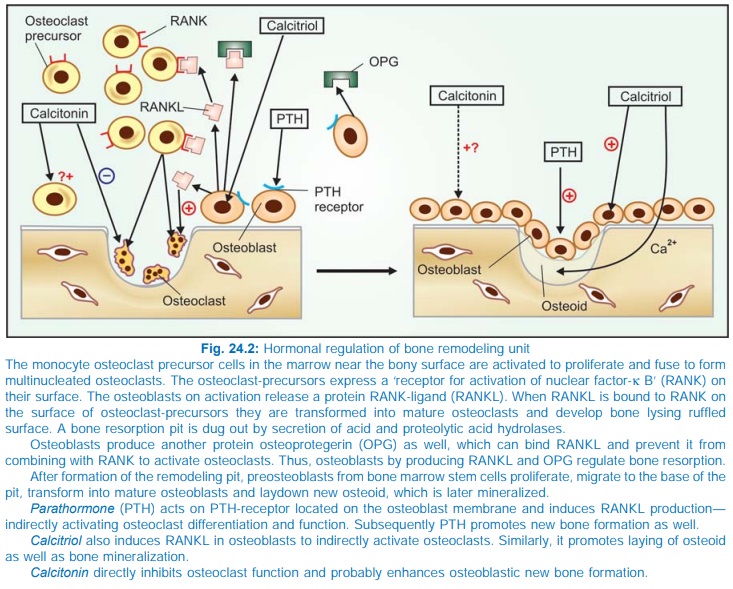
two closely coupled but directionally opposite processes of resorption and new
bone formation (Fig. 24.2). Millions of tiny remodeling units are working on
the surface of bone trabeculae and Haversian canals to dig micropits by
osteoclastic activity and then repair by osteoblastic activity in which first
collagen and other proteins (osteoid) are deposited followed by mineralization;
the full cycle taking 4–6 months. Diet, exercise, several hormones and drugs
regulate the number and efficiency of bone remodeling units at any given time.
Remodeling deficits accumulate over lifetime to account for age related bone
loss, the pace of which can be retarded or accelerated by modulating the above
listed influences. Estrogen lack after menopause mainly causes loss of
trabecular bone, particularly affecting vertebrae, wrist bones and femoral
neck. Minimal trauma/compression fractures are most common at these sites.
Absorption And Excretion
Calcium is absorbed by
facilitated diffusion from the entire small intestine as well as from duodenum
by a carriermediated active transport under the influence of vit D. Phytates,
phosphates, oxalates and tetracyclines complex Ca2+ in an insoluble form in the
intestines and interfere with absorption.
Glucocorticoids and
phenytoin also reduce calcium absorption.
All ionized calcium is
filtered at the glomerulus and most of it is reabsorbed in the tubules. Vit D
increases and calcitonin decreases proximal tubular reabsorption, while PTH
increases distal tubular reabsorption of Ca2+. About 300 mg of endogenous
calcium is excreted daily: half in urine and half in faeces. To maintain
calcium balance, the same amount has to be absorbed in the small intestine from
the diet. Because normally only 1/3rd of ingested calcium is absorbed, the
dietary allowance for calcium is 0.8–1.5 g per day. However, calcium deficiency
and low dietary calcium increases fractional calcium absorption.
Thiazide diuretics
impede calcium excretion by facilitating tubular reabsorption.
Preparations
1. Calcium chloride
(27% Ca): is freely water soluble but highly irritating—tissue necrosis occurs
if it is injected i.m. or extravasation takes place during i.v. injection.
Orally also the solution irritates.
2. Calcium gluconate (9% Ca): is available as 0.5 g and 1 g
tablets and 10% injection (5 ml amp.) It is nonirritating to g.i.t. and the
vascular endothelium—a sense of warmth is produced on i.v. injection: extravasation
should be guarded. It is the preferred injectable salt.
3. Calcium lactate (13% Ca): is given orally, nonirritating and
well tolerated.
4. Calcium dibasic phosphate (23% Ca): is insoluble, reacts with HCl to form soluble chloride in the stomaCh. No. It is bland; used orally as antacid and to supplement calcium.
5. Calcium carbonate (40% Ca): insoluble,
tasteless and nonirritating. It has been used as an antacid—reacts with HCl to
form chloride which may be absorbed from the intestines.
Side Effects
Calcium supplements are usually well tolerated; only
g.i. side effects like constipation, bloating and excess gas (especially with
cal. carbonate) have been reported.
Some Combined Formulations
CALCINOLRB: Cal. carb 0.375 g, Cal. Phos 75 mg + vit D3 250 IU
tab.
CALCIUMSANDOZ: Cal. glucobionate 137.5 mg/ml inj. 10 ml amp., also
tabs containing cal. carbonate 650 mg. KALZANA: Cal. dibasic phos 430 mg + Vit
C and D3 200 IU tab, also syrup: Cal. gluconate 300 mg, Cal. lactobionate 1.1
g, Cal. phos. 75 mg per 5 ml, containing Vit A, C, niacinamide and D3 200 IU.
OSTOCALCIUM: Cal. phos 380 mg + Vit D3 400 IU tab, also syrup:
Cal. phos 240 mg per 5 ml containing Vit D3 200 IU and B12.
SHELCAL: Cal. carb. 625 mg (eq 250 mg elemental cal), Vit D3
125 IU tab and per 5 ml syr.
MACALVIT: Cal. carb. 1.25 g, cholecalciferol 250 IU tab; Cal.
gluconate 1.18 g, Cal. lactobionate 260 mg + Vit D3 100 IU per 5 ml
syr.
CALCIMAX: Cal. carb. (150 mg cal), dibasic cal. phos. (23.3 mg
cal) with magnesium, zinc and vit D3 200 IU tab.; also syrup cal. carb. (150 mg
cal) with magnesium, zinc and vit D3 200 IU per 5 ml syrup.
Use
1. Tetany
For immediate
treatment of severe cases 10–20 ml of Cal.
gluconate (elemental calcium 90–180 mg) is injected i.v. over 10 min, followed by
slow i.v. infusion. A total of 0.450.9 g calcium (50 to 100 ml of cal. gluconate
solution) over 6 hours is needed for completely reversing the muscle spasms.
Supportive treatment with i.v. fluids and oxygen inhalation may be required. Long-term
oral treatment to provide 1–1.5 g of calcium daily is instituted along with
vit. D. Milder cases need oral therapy only.
2. As Dietary Supplement
Especially in growing children, pregnant, lactating and menopausal women.
The dietary allowance recommended by National Institute of Health (1994) is—

Calcium supplement can reduce bone loss in predisposed women as
well as men. It is often given to fracture patients, but if diet is adequate
this does not accelerate healing.
3. Osteoporosis
In the prevention and treatment of osteoporosis with HRT/raloxifene/
alendronate, it is important to ensure that calcium deficiency does not occur.
Calcium + vit D3 have adjuvant role to HRT/raloxifene/bisphosphonates
in prevention and treatment of osteoporosis.
However, the efficacy of calcium ± vit D supplements alone in increasing
bone mass or preventing fractures among menopausal women/elderly men is
controversial. While several studies have reported a reduction in fracture
risk, others have found no benefit. In the recently concluded 7 year
prospective WHI study involving >36000 postmenopausal women (5179 years),
the overall risk of fractures was the same in the calcium (1 g/day) + vit D
(400 IU/ day) group as in the placebo group, though the bone mineral density at
the hip was 1% higher in the treated group. Certain subgroups of osteoporotic subjects
may benefit from calcium supplements, but the benefit appears to be marginal
and limited to cortical bone loss only.
4. Empirically, Cal. gluconate i.v. has been used in dermatoses,
paresthesias, weakness and other vague complaints. Any benefit is probably psychological
due to warmth and other subjective effects produced by the injection.
5. As antacid (see Ch. No. 46).
Related Topics
