Descriptions of Spin Systems
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Structure Determination of Organic Compounds
It is often helpful to categorize spin systems in terms of the chemical and magnetic equivalence of coupled protons. Protons are chemically equivalent if they have the same chemical environment and thus the same chemical shift.
DESCRIPTIONS OF SPIN SYSTEMS
It
is often helpful to categorize spin systems in terms of the chemical and
magnetic equivalence of coupled protons. Protons are chemically equivalent if
they have the same chemical environment and thus the same chemical shift.
Chemical equivalence can result from either identical environments or rapid
rotations which yield an “average” environment for a group of protons.
Considering toluene, it is seen that the two meta protons are found in the
plane of the ring between the ortho and para protons.
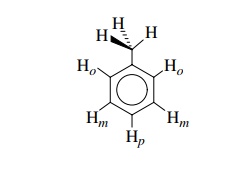
They
have the same chemical environment and thus absorb at the same frequency. The
methyl group is a singlet indicating that the three methyl protons absorb at
the same frequency and thus are chemically equivalent, yet in the conformation
shown it is clear that the environment of each proton is not the same. One is
found in the plane of the ring while a second is above and the third is below
the plane of the ring. However, due to rapid rotation of the methyl group,
these hydrogens rapidly exchange positions and thus all absorb at an “average”
frequency and all are chemically equivalent by rotation.
Protons
are magnetically equivalent if they have the same chemical shift and are
coupled equally to other equivalent nuclei in the molecule. This is simi-lar to
chemical equivalence but is a more rigorous definition of equivalence. For
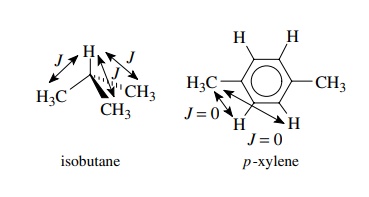
example, the methyl protons of isobutane are chemically and magnetically
equivalent since they absorb at the same frequency and are all coupled equally
to the methine proton (which should be split into a 10-line multiplet!).
Likewise the two methyl groups of p-xylene
are chemically and magnetically equivalent because they are coupled equally (J = 0) to the aromatic protons both
ortho and meta to them.
On
the other hand, the ortho protons of o-dibromobenzene
are chemically equivalent because they have the same environment, but they are
magnetically nonequivalent since a given ortho proton is not coupled equally to
the two meta protons (there is a 1,2 interaction with one and a 1,3 interaction
with the other and J1,2 = J1,3). By the same
arguments the methylene groups of 1,4-dibromo-cis-2-butane are chemically equivalent but magnetically
nonequivalent because each methylene group is not coupled equally to the
chemically equivalent vinyl hydro-gens (again J1,2 = J1,3). Of course this also means
that the vinyl hydrogens are magnetically nonequivalent since a given vinyl
hydrogen is not equally coupled to the two methylene groups.
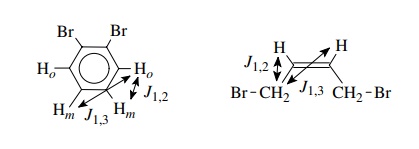
We
would expect that the spectrum of the latter compound would consist of two
signals: a two-proton triplet in the vinyl region and a four-proton doublet in
the allylic region. This is because the coupling constant J1,3 is zero.
It if were not zero, then a more complicated spectrum would result. Thus
magnetic nonequivalence can lead to much more complicated spectra.
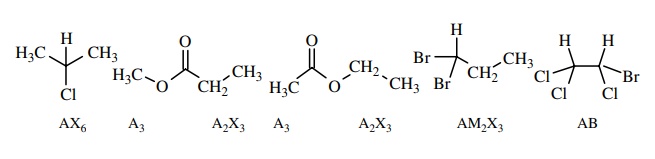
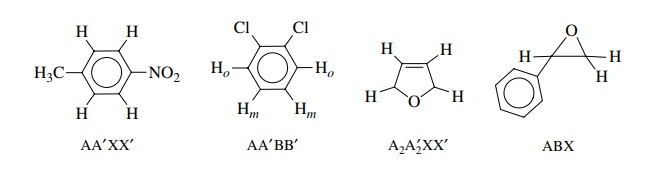
Using
chemical and magnetic equivalence, it is possible to designate the num-ber and
type of different protons in a spin system. This is done by choosing letters of
the alphabet to indicate protons of similar chemical shift: ABC or MNO or XYZ.
For only two types of protons, letters from the first part and last past of the
alphabet are chosen (A, X). If three types are present, then letters from the middle
group are also used (e.g., A, M, X). A subscript is used to indicate how many
of each type of protons is present in the spin system. Several spin systems are
shown below with their designations. All are examples of groups of chem-ically
and magnetically equivalent protons. A molecule can contain more than one spin
system if they are isolated from each other.
Protons
that are chemically equivalent but magnetically nonequivalent are indi-cated
by, for example, AA . The examples of such systems given below illustrate the
method. This system for designating spin systems is merely a labeling device.
The appearance of actual spectra will depend on the magnitude of the various J values.
Nevertheless this is a convenient and common way of categorizing coupled proton
systems.
Another
structural factor which can lead to nonequivalence of aliphatic protons is the
symmetry properties of protons:
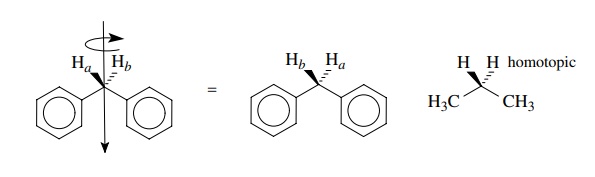
1.
Aliphatic protons which are interconvertible by a rotational axis are termed
homotopic and are chemically and magnetically equivalent. For example, the
methylene protons of diphenylmethane are homotopic, as are the methy-lene
protons and the methyl protons of propane.
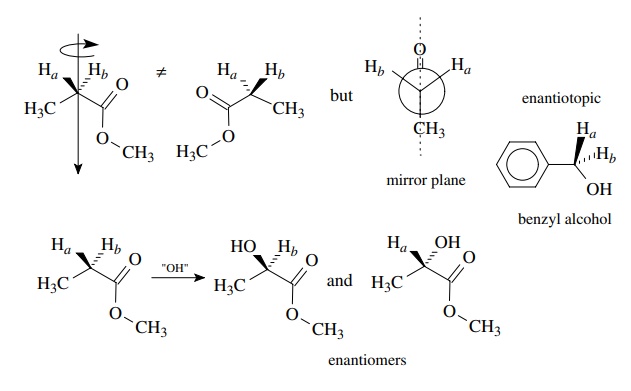
2.
Methylene protons which are not interconvertible by rotation but are
inter-convertible by reflection through a plane of symmetry are enantiotopic
and are chemically and magnetically equivalent in an achiral environment.
Alternatively protons are enantiotopic if sequential replacement by a
dif-ferent group gives a pair of enantiomers. The methylene protons of methyl
propionate are enantiotopic because they are interchangeable by reflection but
not rotation. (The protons of both methyl groups are interchangeable by
rotation and are thus homotopic.) Replacement of Ha and Hb
by another group such as hydroxy gives R-
and S-methyl lactate, respectively.
The benzylic protons of benzyl alcohol are enantiotopic by the same criteria.
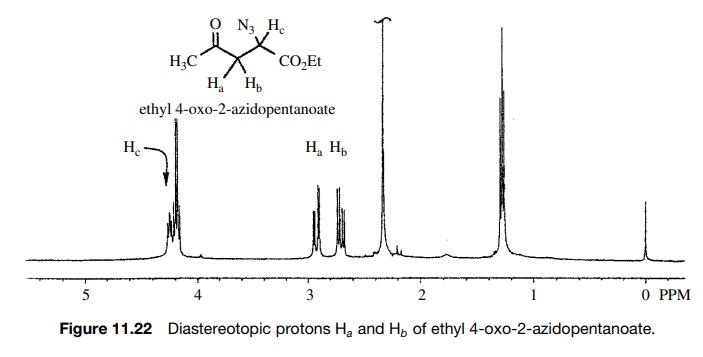
3. Methylene protons which are not interconvertible by either reflection or rotation are diastereotopic and are chemically and magnetically nonequivalent.
The presence of one or more chiral centers in a molecule leads to
diastereotopic methylene groups since the replacement of each proton by another
group gives a pair of diastereomers. Since diastereotopic protons are not
related by symmetry, they have unique environments and thus unique chemical
shifts and coupling constants.
The
C-2 methylene protons Ha
and Hb in
ethyl-3-azido-4-oxopentanoate are diastereotopic because of the chiral center
at C-3 (Figure 11.22). The protons Ha
and Hb have slightly
different chemical shifts and split each other, and they are not coupled
equally to the C-3 methine proton Hc
. Thus Ha and Hb split each other into an AB
quartet, which is further split into doublets by Hc . Note that the splitting for each proton of the AB
quartet has a different coupling constant with Hc . Although Hc
is slightly obscured by the CH2 protons of the ethyl group, it can
be seen that the signal for this proton is not a triplet but rather looks like
a doublet of doublets, as expected from the fact that Jac ≠
Jbc .
Related Topics
