Diagnosis of Renal Adverse Drug Reactions
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Renal Adverse Drug Reactions
None of the described functional or morphologic alterations to the kidney are pathognomonic to ADR.
DIAGNOSIS OF RENAL ADVERSE DRUG
REACTIONS
None
of the described functional or morphologic alterations to the kidney are
pathognomonic to ADR. So, general principles of renal diagnostic procedures
apply to the evaluation of adverse renal drug reactions.
Although
glomerular and tubular processes coop-erate in renal excretory function, renal
function is routinely expressed as GFR or creatinine clear-ance. Measurement of
creatinine clearance requires a 24-hour urine collection, which is cumbersome
and prone to error. Therefore, it is now generally accepted to calculate
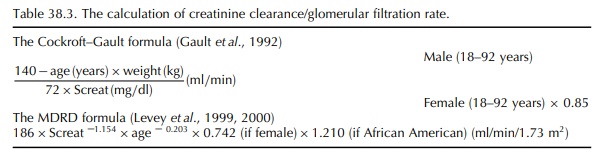
creatinine clearance using nomo-grams like the Cockroft–Gault formula (Cockroft
and Gault, 1976; Gault et al., 1992)
or the MDRD formula (Levey et al.,
1999, 2000) (Table 38.3). Care must be taken always to compare the result of
the creatinine clearance calculation to an age- and gender-matched population
(Elseviers et al., 1987).
The
determination of renal function by means of the creatinine clearance, however,
remains a poorly sensitive method of monitoring the kidney func-tion.
Therefore, in experimental settings, a more accurate way of assessing changes
in GFR is to measure the clearance of a compound that is freely filtered by the
glomerulus but is neither secreted nor absorbed by the tubules. Radiolabeled
sodium iodothalamate and ethylenediaminetetraacetic acid (EDTA) are substances
commercially available for this purpose.
The most common urinary biomarker used in renal diagnosis is proteinuria. Under normal conditions, the glomerular filtration barrier restricts the transfer of high molecular weight proteins from the plasma to the lumen of the tubule. High molecular weight proteins appearing in the urine points to a pathological condi-tion of the glomerulus, changing the permselectiv-ity of the filter. Under normal conditions, a minute amount of low molecular weight proteins are filtered, which then undergo endocytic reabsorption by prox-imal tubular cells. When the reabsorptive capacity of the proximal tubule is compromised, low molecu-lar weight proteins appear in the urine in measurable amounts. Determination of the quantity and the quality of urinary proteins allows for the distinction between ‘glomerular’ and ‘tubular’ proteinuria.
Enzymuria
has been extensively used by toxi-cologists to detect early renal damage.
Urinary enzymes bear the potential of determining the site of damage because
different enzymes are localised in specific segments of the nephron. For
exam-ple, alanine aminopeptidase, alkaline phosphatase and glutamyltransferase
are enzymes bound to the brush border of proximal tubular cells. Their
appearance in the urine should be indicative for turnover of brush border. The
general acceptance of urinary enzyme excretion as a measure of tubular
dysfunction in human safety studies has been limited for several reasons.
First, it has been impossible to link the pres-ence of the different enzymes
appearing in the urine to specific tubular disease states. Secondly, a
relation-ship between the magnitude of the enzymuria and the severity of
tubular injury has not been established. Furthermore, enzymuria may occur in
normal situa-tions due to increased brush border turnover, altered membrane
permeability or increased synthesis.
In
general a renal biopsy is not needed to estab-lish the diagnosis of a renal
adverse event. When a glomerulopathy is suspected, only a biopsy allows to
distinguish between the different histopathologic types. Ideally, the diagnosis
of acute interstitial nephritis is also confirmed by histopathologic
examination.
Presently,
during drug development, preclinical toxicity tests involve the use of animal
models. However, advances in cell and tissue culture will permit the
development of in vitro toxicity
assays. The aim of the development of in
vitro tests is not only to replace in
vivo animal testing but also to study the mechanisms of cell modulation by
toxic compounds. Recently, for example, in
vitro studies involving renal cells in culture suggested that the
underlying mechanism of the proteinuria associated with the use of rosuvastatin
was inhibition by the statin of the endocytotic uptake of proteins by the
proximal tubular cell (Verhulst, D’Haese and De Broe, 2004). Several permanent
and immortalised cell lines of human and non-human origin are available,
offer-ing several advantages over primary cultures such as an unlimited life
span and the lack of time-consuming isolation procedures. The most widely used
renal epithelial cell lines of animal origin are the LLC-PK1 (Hampshire pig)
and OK (American opossum) cell lines, exhibiting characteristics suggestive of
proxi-mal tubular origin, and the MDCK (Cocker Spaniel) cell line, exhibiting
characteristics suggestive of distal origin.
Related Topics
