Factors Affecting Choice of Antimicrobial Agent
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Chemical Disinfectants, Antiseptics And Preservatives
Choice of the most appropriate antimicrobial compound for a particular purpose depends on many factors and the key parameters are described.
FACTORS AFFECTING CHOICE OF ANTIMICROBIAL AGENT
Choice of the most appropriate antimicrobial compound for a particular
purpose depends on many factors and the key parameters are described.
a) Properties Of The Chemical Agent
The process of killing or inhibiting the growth of microorganisms using
an antimicrobial agent is basically that of a chemical reaction and the rate
and extent of this reaction will be influenced by concentration of agent,
temperature, pH and formulation. Tissue toxicity influences whether a chemical
can be used as an antiseptic or preservative, and this limits the range of
agents for these applications or necessitates the use of lower concentrations
of the agent.
b) Microbiological Challenge
The types of microorganism present and
the levels of microbial contamination (the bioburden) both have
a significant effect on the outcome of treatment. If the bioburden is high,
long exposure times and/or higher concentrations of antimicrobial may be
required. Microorganisms vary in their sensitivity to the action of chemical
agents. Some organisms merit attention either because of their resistance to
disinfection or because of their significance in cross-infection or nosocomial
(hospital-acquired) infections. Of particular concern is the significant
increase in resistance to disinfectants resulting from microbial growth in
biofilm form rather than free suspension. Microbial biofilms form readily on
available surfaces, posing a serious problem for hospital infection control
committees in advising suitable disinfectants for use in such situations.
The efficacy of an antimicrobial agent
must be investigated by appropriate capacity, challenge and in-use tests to
ensure that a standard is obtained which is appropriate to the intended use. In
practice, it is not usually possible to know which organisms are present on the
articles being treated. Thus, it is necessary to categorize agents according to
their antimicrobial activity and for the user to be aware of the level of antimicrobial
action required in a particular situation.
i) Vegetative bacteria
At in-use concentrations, chemicals
used for disinfection should be capable of killing bacteria and other organisms
expected in that environment within a defined contact period. This includes ‘problem’
organisms such as methicillin-resistant Staphylococcus aureus (MRSA),
vancomycin-resistant enterococci (VRE) and species of Listeria, Campylobacter and Legionella. Antiseptics and preservatives are also
expected to have a broad spectrum of antimicrobial activity but at their in-use
concentrations, after exerting an initial biocidal (killing) effect, their main
function may be biostatic (inhibitory). Gram-negative bacilli, which are a
major causes of nosocomial infections, are often more resistant than
Gram-positive species. Pseudomonas aeruginosa, an
opportunistic pathogen has gained a reputation as the most resistant of the
Gram-negative organisms. However, problems mainly arise when a number of
additional factors such as heavily soiled articles or diluted or degraded
disinfectant solutions are employed.
ii)
Mycobacterium tuberculosis
M. tuberculosis (the tubercle bacillus) and other mycobacteria are resistant to
many bactericides. Resistance is either (1) intrinsic, mainly due to reduced
cellular permeability or (2) acquired, due to mutation or the acquisition of
plasmids . Tuberculosis remains an important public health hazard, and indeed
the annual number of tuberculosis cases is rising in many countries. The
greatest risk of acquiring infection is from the undiagnosed patient. Equipment
used for respiratory investigations can become contaminated with mycobacteria
if the patient is a carrier of this organism. It is important to be able to
disinfect the equipment to a safe level to prevent transmission of infection to
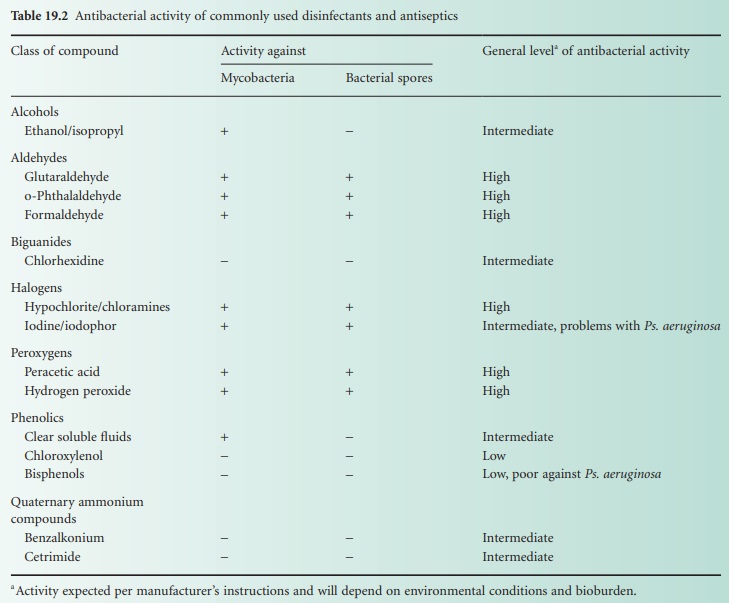
other patients (Table 19.2).
iii)
Bacterial spores
Bacterial spores can exhibit
significant resistance to even the most active chemical disinfectant treatment.
The majority of disinfectants have no useful sporicidal action in a
pharmaceutical context, which relates to disinfection of materials, instruments
and environments that are likely to be contaminated by the spore-forming
genera Bacillus and Clostridium.
However, certain aldehydes, halogens and per-oxygen compounds display very good
activity under controlled conditions and are sometimes used as an alternative
to physical methods for sterilization of heat-sensitive equipment. In these
circumstances, correct usage of the agent is of paramount importance, as safety
margins are lower in comparison with physical methods of sterilization .
Clostridium difficile is a particularly problematic contaminant in hospital
environments, resulting in high levels of morbidity and mortality. In addition
to stringent hand-washing, meticulous environmental disinfection procedures
must be in place, e.g. using solutions of 5.25–6.15% sodium hypochlorite for
routine disinfection. When high-level disinfection of Cl. difficile is required, 2% glutaraldehyde,
0.55% o-phthalaldehyde and 0.35% peracetic acid are effective.
The antibacterial activity of some
disinfectants and antiseptics is summarized in Table 19.2.
iv)
Fungi
The vegetative fungal form is often as
sensitive as vegetative bacteria to chemical antimicrobial agents. Fungal
spores (conidia and chlamydospores) may be more resistant, but this resistance
is of much lesser magnitude than that exhibited by bacterial spores. The ability
to rapidly destroy pathogenic fungi such as the important nosocomial
pathogen Candida albicans, filamentous fungi such as Trichophyton mentagrophytes, and spores of common
spoilage moulds such as Aspergillus niger is
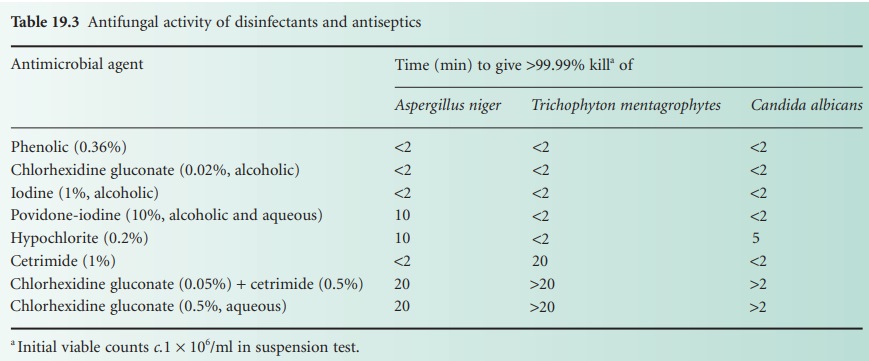
put to advantage in many applications of use. Many disinfectants have good
activity against these fungi (Table 19.3).
In addition, ethanol (70%) is rapid and reliable against Candida species.
v)
Viruses
Susceptibility of viruses to antimicrobial agents can depend on whether
the viruses possess a lipid envelope. Non-lipid viruses are frequently more resistant
to disinfectants and it is also likely that such viruses cannot be readily
categorized with respect to their sensitivities to antimicrobial agents. These
viruses are responsible for many nosocomial infections, e.g. rotaviruses,
picornaviruses and adenoviruses and it may be necessary to select an antiseptic
or disinfectant to suit specific circumstances. Certain viruses, such as Ebola
and Marburg, which cause haemorrhagic fevers, are highly infectious and their
safe destruction by disinfectants is of paramount importance. Hepatitis A is an
enterovirus considered to be one of the most resistant viruses to disinfection.
There is much concern for the safety of
personnel handling articles contaminated with pathogenic viruses such as hepatitis
B virus (HBV) and HIV. Disinfectants must be able to treat rapidly and reliably
accidental spills of blood, body fluids or secretions from HIV-infected
patients. Such spills may contain levels of HIV as high as 104 infectious units/ml. Fortunately, HIV is
inactivated by most chemicals at in-use concentrations. However, the
recommendation is to use high-level disinfectants (see Table 19.2)
for decontamination of HIV-or HBV-infected reusable medical equipment. For patient
care areas, cleaning and disinfection with intermediate-level disinfectants is
satisfactory. Flooding with a liquid germicide is required only when large
spills of cultured or concentrated infectious agents have to be dealt with.
The World Health Organization (WHO) and epidemiologists in many
countries track outbreaks of influenza, especially in relation to potential
epidemic and pandemic situations arising. The H1N1 outbreak in 2009 generated
considerable concern. As an influenza A virus, however, it is susceptible to a
large number of disinfectant products when they are used on hard, non-porous
surfaces that may be contaminated. Although no research has been conducted on
the susceptibility of 2009 H1N1 influenza virus to chlorine and other disinfectants
in swimming pools and spas, studies have demonstrated that free chlorine levels
of 1–3 mg/L (1–3 ppm) are adequate to disinfect avian H5N1 influenza virus.
vi)
Protozoa
Acanthamoeba spp. can cause acanthamoeba keratitis with associated corneal
scarring and loss of vision in wearers of soft contact lenses. The cysts of
this protozoan present a particular problem in respect of lens disinfection.
The chlorine-generating systems in use are generally inadequate. Polyaminopropyl
biguanide with or without chlorhexidine (0.003%) and polyhexamethylene
biguanide (0.0005%) both show ability as an acanthamoebicide in combating 103levels of cysts. Hydrogen peroxide-based
disinfection is considered completely reliable and consistent in producing an
acanthamoebicidal effect.
vii)
Prions
Prions are generally considered to be
the infectious agents most resistant to chemical disinfectants and
sterilization processes; strictly speaking, however, they are not
microorganisms because they have no cellular structure nor do they contain
nucleic acids. As small proteinaceous infectious particles they are a unique
class of infectious agent causing spongiform encephalopathies such as bovine
spongiform encephalopathy (BSE) in cattle and Creutzfeldt–Jakob disease (CJD)
in humans. There is considerable concern about the transmission of these agents
from infected animals or patients. Risk of infectivity is highest in brain,
spinal cord and eye tissues. Prions are considered resistant to most disinfectant
procedures. For heat-resistant medical instruments that come into contact with
high infectivity tissues or high-risk contacts, immersion in sodium hydroxide
(1 M) or sodium hypochlorite (20 000 ppm available chlorine) for 1 hour is
advised in WHO guidelines and this must be followed by further treatment
including autoclaving, cleaning and routine sterilization. Recently, a formulation
of 0.2% sodium docecyl sulphate, 0.3% NaOH in 20% n-propanol, has achieved potent
decontamination of steel carriers contaminated with PrPTSE, the biochemical marker
for prion infectivity, from 263K scrapie hamsters (5.5 log10 units reduction) or patients with sporadic or
variant Creutzfeldt–Jacob disease. No low-temperature sterilization technology
is effective.
c) Intended Application
The intended application of the antimicrobial agent, whether for
preservation, antisepsis or disinfection, will influence its selection and also
affect its performance. For example, in medicinal preparations the ingredients
in the formulation may antagonize preservative activity. The risk to the
patient will depend on whether the antimicrobial is in close contact with a
break in the skin or mucous membranes or is introduced into a sterile area of the
body.
In disinfection of instruments, the chemicals used must not adversely
affect the instruments, e.g. cause corrosion of metals, affect clarity or
integrity of lenses, or change the texture of synthetic polymers. Many
materials such as fabrics, rubber and plastics are capable of adsorbing certain
disinfectants, e.g. quaternary ammonium compounds (QACs) are adsorbed by
fabrics, while phenolics are adsorbed by rubber, the consequence of this being
a reduction in the concentration of active compound. A disinfectant can only
exert its effect if it is in contact with the item being treated. Therefore,
access to all parts of an instrument or piece of equipment is essential. For
small items, total immersion in the disinfectant must also be ensured.
d) Environmental Factors
Organic matter can have a drastic
effect on antimicrobial capacity either by adsorption or chemical inactivation,
thus reducing the concentration of active agent in solution or by acting as a
barrier to the penetration of the disinfectant. Blood, body fluids, pus, milk,
food residues or colloidal proteins, even when present in small amounts, all
reduce the effectiveness of antimicrobial agents to varying degrees, and some
are seriously affected. In their normal habitats, microorganisms have a
tendency to adhere to surfaces and are thus less accessible to the chemical
agent. Some organisms are specific to certain environments and their
destruction will be of paramount importance in the selection of a suitable
agent, e.g. Legionella in cooling towers
and non-potable water supply systems, Listeria in the
dairy and food industry and HBV in blood-contaminated articles.
Dried organic deposits may inhibit penetration of the chemical agent.
Where possible, objects to be disinfected should be thoroughly cleaned. The
presence of ions in water can also affect activity of antimicrobial agents,
thus water for testing biocidal activity can be made artificially ‘hard’ by
addition of ions.
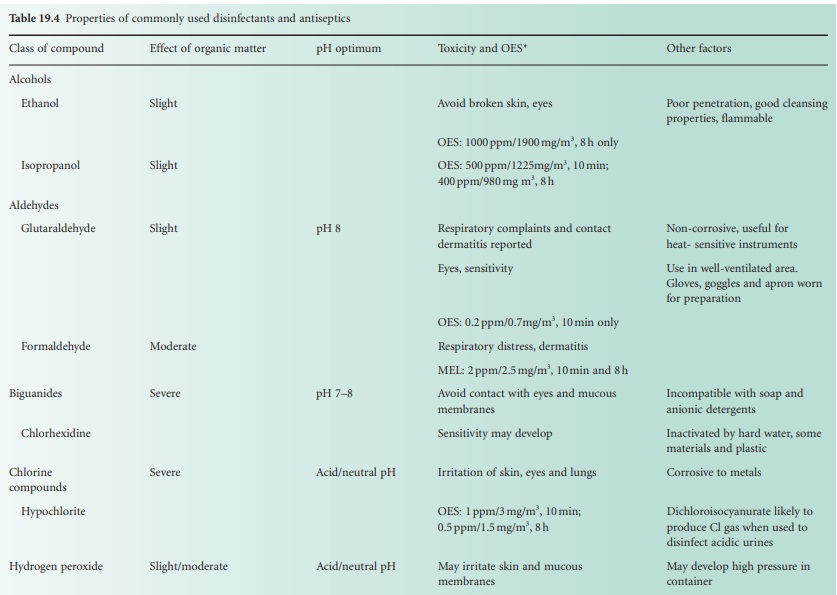
These factors can have very significant
effects on activity and are summarized in Table 19.4.

e) Toxicity Of The Agent
In choosing an antimicrobial agent for
a particular application some consideration must be given to its toxicity.
Increasing concern for health and safety is reflected in the Control of
Substances Hazardous to Health (COSHH) Regulations that specify the precautions
required in handling toxic or potentially toxic agents. In respect of disinfectants
these regulations affect, particularly, the use of phenolics, formaldehyde and
glutaraldehyde. Toxic volatile substances, in general, should be kept in
covered containers to reduce the level of exposure to irritant vapour and they
should be used with an extractor facility. Limits governing the exposure of individuals
to such substances are now listed, e.g. 0.7 mg/m3 (0.2 ppm)
glutaraldehyde for both short-and long-term exposure. Many disinfectants
including the aldehydes, glutaraldehyde less so than formaldehyde, may affect
the eyes, skin (causing contact dermatitis) and induce respiratory distress.
Face protection and impermeable nitrile rubber gloves should be worn when using
these agents. Table 19.4 lists
the toxicity of many of the disinfectants in use and other concerns of toxicity
are described below for individual agents.
The COSHH Regulations specify certain disinfectants that contain active
substances not supported under the BPD that had to be phased out by 2006. Specified
disinfection procedures applied to laboratories in relation to spills and routine
use state that certain phenolic agents (including 2,4,6-trichlorophenol and xylenol)
can no longer be employed in disinfectant products.
Because of the historically high number
of occupational asthma cases caused by glutaraldehyde (an alkylating agent)
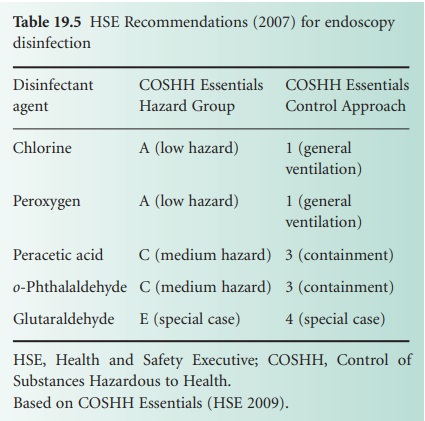
products in chemical disinfection of endoscopes, an HSE report (2007) sought alternatives
to this agent. The report recommended the preferential use of an oxidizing
agent such as a chlorine-based or peroxygen-based product rather than a product
containing an alkylating agent. However, it was recognized that consideration
must be given to incompatibility of disinfectants with endoscope construction
materials in some cases (Table 19.5).
In all situations where the atmosphere of a workplace is likely to be
contaminated by disinfectant, sampling and analysis of the atmosphere may need
to be carried out on a periodic basis with a frequency determined by
conditions.
Related Topics
