Non-Antibiotic Antimicrobial Agents: Mode of Action and Resistance
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Non-Antibiotic Antimicrobial Agents: Mode Of Action And Resistance
The group of agents which comprises antiseptics, disinfectants, chemical sterilants and preservatives (often collectively called biocides) have frequently been classified as non-specific protoplasmic poisons. Such a broad generalization is, however, far from the true position.
NON-ANTIBIOTIC
ANTIMICROBIAL AGENTS: MODE OF ACTION AND RESISTANCE
INTRODUCTION
The group of agents which comprises antiseptics, disinfectants, chemical
sterilants and preservatives (often collectively called biocides) have
frequently been classified as non-specific protoplasmic poisons. Such a broad
generalization is, however, far from the true position.
It is often convenient to consider the
modes of action of biocides in terms of their targets within the bacterial
cell, in particular the region of the cell in which their activity is deemed to
predominate. Thus agents have been described variously as cell wall, membrane
and cytoplasm-active. This characterization, whilst having the benefits of
simplicity, does not necessarily describe their mechanism of action; this is
best classified by effects on functional structures and cellular processes. The
range and complexity of the reactions involved will become apparent from this
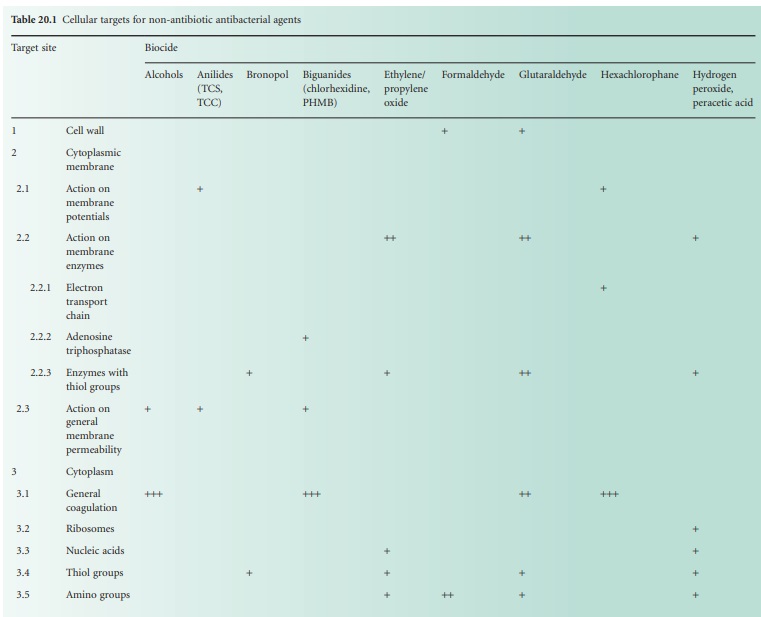
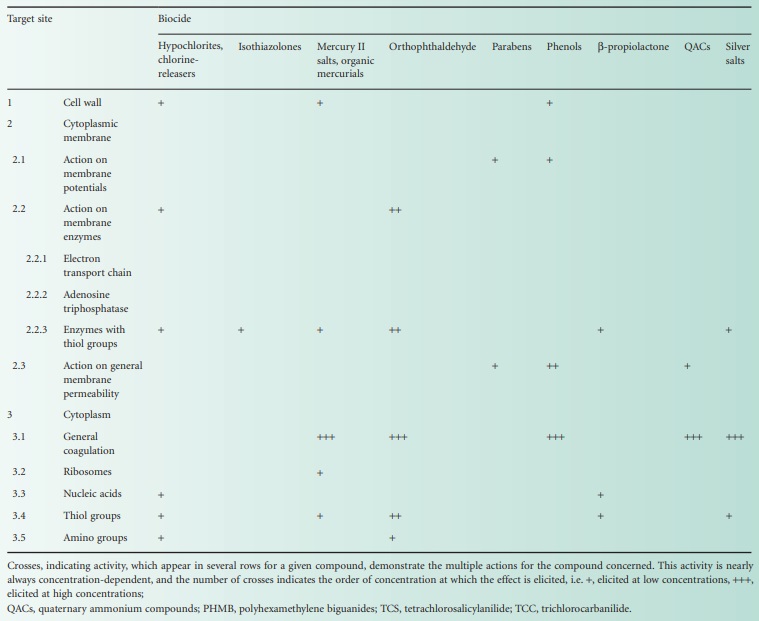
account and Table 20.1,
and it is worth emphasizing here that many of these substances exhibit
concentration-dependant dual or even multiple effects.
Mechanisms Of Interaction
For a chemical to exhibit antimicrobial
activity it usually has to undergo a sequence of events that begins with
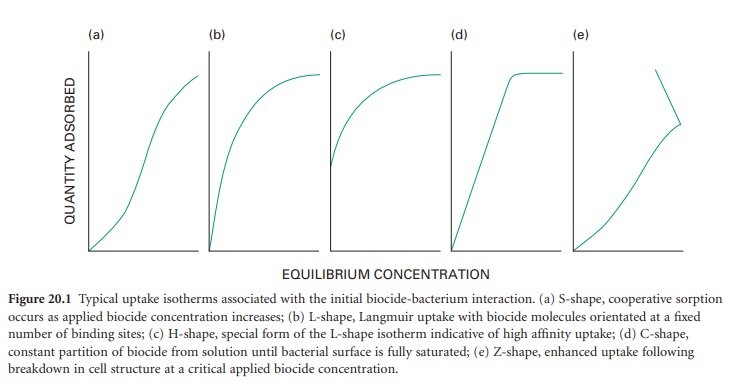
adsorption on to the microbial cell surface. This initial uptake is a physicochemical
phenomenon which can be generally characterized into one of several uptake
isotherms (Figure 20.1);
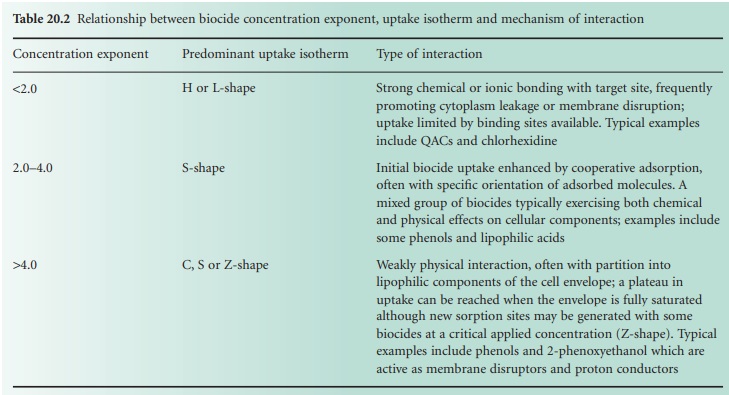
it bears a relationship to the concentration exponent which describes the influence of concentration
on activity (Table 20.2).
In the many cases where the chemical has an intracellular site of action, adsorption
must be followed by passage through porin channels in Gram-negative cells,
diffusion across, or into, the lipid-rich cytoplasmic membrane, and finally,
interaction with proteins, enzymes, nucleic acids or other targets within the
cytoplasm. These processes are markedly influenced by the physicochemical
characteristics of the biocide, e.g. ionization constant and lipid solubility,
so the wide diversity of structures exhibited by biocide molecules complicates
the prediction of antimicrobial potency and explanation of their mechanisms of
action. Despite this, it is important to recognize that there is a basis upon
which the mode of action might be deduced, because there are certain molecular
features of biocides that are associated with activity against particular
cellular targets.
Antimicrobial Effects
Antimicrobial activity is often strongly influenced by the affinity of
the biocide for structural or molecular components of the cell, and this, in
turn, may depend upon the attraction of dissimilar charges or on hydrophobic
interactions. Antimicrobial drugs whose active species is positively charged,
e.g. quaternary ammonium compounds (QACs) and chlorohexidine, display an
affinity for the negative charges of sugar residues on the microbial cell
surface or phosphate groups on the membrane(s); adsorption of these biocides,
and thus their antimicrobial activity, is increased as the pH rises and the
cell surface becomes more electronegative. Antimicrobial chemicals possessing a
long alkyl chain, on the other hand, may integrate into the hydrophobic region
of phospholipid molecules within the membrane and so cause membrane disruption
and fatal permeability changes. Further examples of structure-activity
relationships are afforded by aldehydes, particularly glutaraldehyde, which is
an electrophile that is able to react with molecules possessing thiol (SH) or
amino groups, e.g. proteins. This reaction, too, increases with pH, so
aldehydes are more active in alkaline conditions. Biocides containing heavy
metal ions, e.g. silver or mercury, also damage or inactivate enzymes and
structural proteins by virtue of interactions with thiol groups. A number of
phenols and bisphenols incorporate a hydroxyl group that is capable of
generating a labile proton, i.e. they are weak acids. A weakly acidic nature
combined with significant lipid solubility are properties associated with
uncoupling agents, i.e. those molecules that can disrupt the proton-motive
force that is responsible for oxidative phosphorylation in the cell. It is
thought that these molecules dissolve in the lipid bilayer of the membrane and
act as proton conductors by virtue of their ionizability. This property,
possessed by biocides such as phenoxythanol and fentichlor, results in the
failure of many important energy-requiring processes in the cell, including the
concentration and retention of sugars and amino acids.
Related Topics
