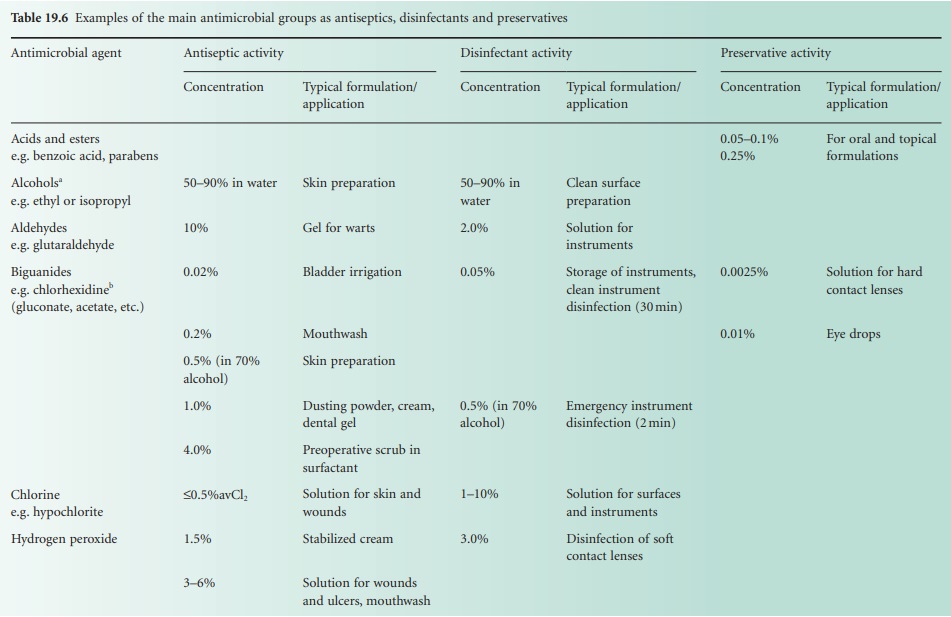
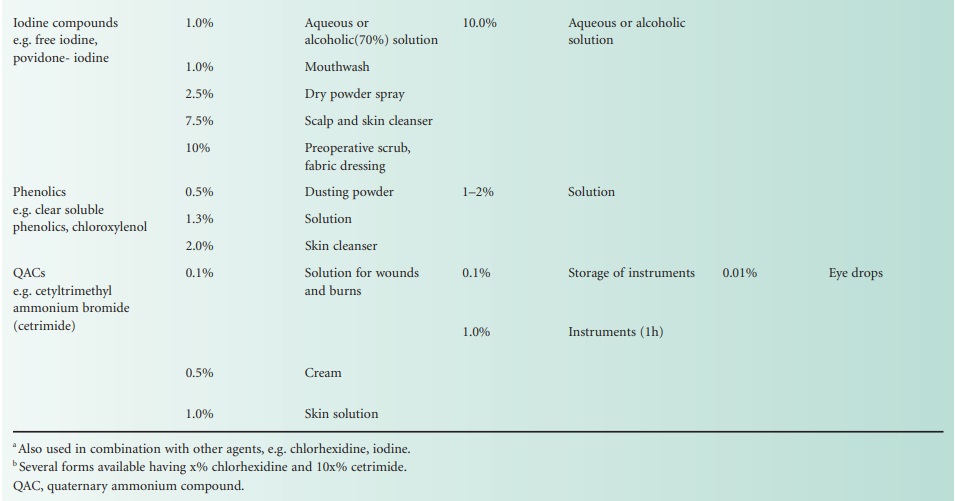
Types of Compound
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Chemical Disinfectants, Antiseptics And Preservatives
The following section presents, in alphabetical order by chemical grouping, the agents most often employed for disinfection, antisepsis and preservation.
TYPES OF COMPOUND
The following section presents, in alphabetical order by chemical grouping, the agents most often employed for disinfection, antisepsis and preservation. This information is summarized in Table 19.6.
A) Acids And Esters
Antimicrobial activity,
within a pharmaceutical context, is generally found only in the organic
acids. These are weak acids and will, therefore, dissociate incompletely to give the three
entities HA, H+ and A− in solution. As the undissociated
form, HA, is the active
antimicrobial agent, the ionization constant,
Ka, is important and the pKa of the
acid must be considered, especially in formulation of the agent.
i) Benzoic acid
This is an organic
acid, C6H5COOH, which
is included, alone or in combination with other preservatives, in many pharmaceuticals. Although the compound is often used as the sodium
salt, the non-ionized acid is the active substance. A limitation on its use is imposed
by the pH of the final product
as the pKa of benzoic acid is 4.2 at which pH 50% of the acid
is ionized. It is advisable to limit use of the acid to preservation of pharmaceuticals with a maximum
final pH of 5.0 and if possible less than 4.0. Concentrations of 0.05–0.1% are suitable for oral preparations. A disadvantage of the compound is the development of resistance by some organisms, in some cases involving metabolism of the acid resulting
in complete loss of activity.
Benzoic acid also has some use in combination with other agents, salicylic
acid for example, in the treatment of superficial fungal infections.
ii)
Sorbic acid
This compound is a widely
used preservative as the acid or
its potassium salt.
The pKa is 4.8 and,
as with benzoic acid, activity decreases with increasing pH and ionization. It is most
effective at pH 4 or below. Pharmaceutical products such as gums, mucilages and syrups are
usefully preserved
with this agent.
iii)
Sulphur dioxide, Sulphites and Meta bisulphites
Sulphur dioxide
has extensive use as a preservative in the food and beverage industries. In a pharmaceutical context, sodium sulphite and
metabisulphite or bisulphite have a dual role,
acting as preservatives and antioxidants.
iv)
Esters of p-hydroxybenzoic acid (parabens)
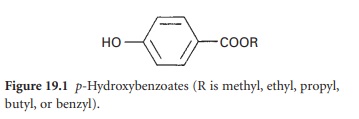
A series
of alkyl esters
(Figure 19.1) of p-hydroxybenzoic acid was originally
prepared to overcome the marked pH dependence on activity of the acids.
These parabens, the methyl, ethyl,
propyl and butyl esters, are less readily ionized, having pKa values in the range 8–8.5,
and exhibit good preservative activity even at pH levels of
7–8, although optimum
activity is again displayed in acidic
solutions. This broader
pH range allows
extensive and successful use of the parabens as pharmaceutical preservatives. They are active
against a wide
range of fungi but are less
so against bacteria, especially the pseudomonads which may
utilize them as a carbon
source. They are frequently used as preservatives of emulsions, creams and lotions where two phases exist.
Combinations of esters are most successful for this type
of product in that
the more water-soluble methyl ester (0.25%) protects the aqueous phase, whereas
the propyl or butyl esters (0.02%) give protection to the oil phase. Such
combinations are also
considered to extend
the range of activity.
As inactivation of parabens occurs
with non-ionic surfactants due care should be taken in formulation with both materials.
B) Alcohols
i) Alcohols Used For Disinfection And
Antisepsis
The aliphatic alcohols, notably ethanol and
isopropanol, are used
for disinfection and antisepsis. They
are bactericidal against vegetative forms, including Mycobacterium
species, but are not sporicidal. Overall cidal activity
drops sharply below 50% concentration.
Alcohols have poor penetration of organic matter
and their use is, therefore, restricted to clean conditions. They possess properties such as a cleansing action
and volatility, are able to achieve a rapid
and large reduction in skin flora
and have been widely
used for skin preparation before
injection or other surgical procedures. The risk
of transmission of infection due to poor
hand hygiene has been attributed to lack of compliance with hand-washing procedures. An alcohol hand-rub offers
a rapid, easy-to-use alternative that is more acceptable to personnel and is frequently recommended for routine
use. However, the contact time of an alcohol-soaked swab with the
skin prior to venepuncture is so brief that
it is thought to be of doubtful value.
Ethanol (CH3CH2OH) is widely used as a disinfectant and antiseptic. The presence of water is essential for activity, hence 100% ethanol is relatively
ineffective. Concentrations between 60% and 95% are bactericidal and a 70% solution is usually employed
for the disinfection of skin,
clean instruments or surfaces. At higher concentrations, e.g. 90%, ethanol
is also active
against fungi and most lipid-containing viruses,
including HIV, though
less so against non-lipid-containing viruses.
Ethanol is also a popular
choice in pharmaceutical preparations and
cosmetic products as a solvent
and preservative, but it is not recommended for cleaning class II recirculating safety cabinets; ethanol
vapours are flammable and the lower explosive limit (LEL) is easily attained. Mixtures with other disinfectants, e.g. with formaldehyde (100 g/L), are more effective than alcohol alone.
Isopropyl alcohol
(isopropanol, CH3.CHOH.CH3) has slightly greater bactericidal activity
than ethanol but is
also about twice as toxic.
It is less active against
viruses, particularly non-enveloped viruses,
and should be considered a limited-spectrum viricide. Used at concentrations of 60–70%,
it is an acceptable alternative to ethanol for preoperative skin treatment and is also employed as a preservative for cosmetics.
ii) Alcohols as preservatives
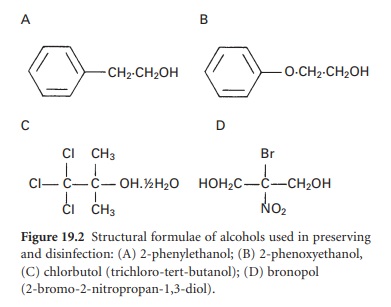
The aralkyl
alcohols and more highly substituted aliphatic alcohols (Figure 19.2)
are used mostly
as preservatives. These include:
•
Benzyl alcohol (C6H5CH2OH). This has antibacterial
and weak local
anaesthetic properties and is used as an antimicrobial preservative at a
concentration of 2%, although its use in cosmetics is restricted.
•
Chlorbutol (chlorobutanol; trichlorobutanol; trichlorot-butanol) is typically
used at a concentration of 0.5%
and is employed as a preservative in injections and eye drops. It is unstable, decomposition occurring at acid pH
during autoclaving, while alkaline solutions are unstable at room temperature.
•
Phenylethanol (phenylethyl alcohol; 2-phenylethanol), having a typical in-use concentration of 0.25–0.5%, is reported to have greater
activity against Gram-negative organisms and is usually
employed in conjunction with another agent.
Phenoxyethanol (2-phenoxyethanol). Typical in-use
concentration: 1%. It is more active
against Ps. Aeruginosa than against
other bacteria and is usually
combined with other preservatives such as the
hydroxybenzoates to broaden the spectrum of antimicrobial activity.
•
Bronopol (2-bromo-2-nitropropan-1,3-diol). Typical in-use concentration: 0.01–0.1%. It has a broad spectrum of antibacterial activity,
including Pseudomonas species. The main limitation on the use of bronopol
is that when exposed to light at alkaline
pH, especially if accompanied
by an increase in temperature, solutions
decompose, turning yellow
or brown. A number of decomposition
products including formaldehyde are produced. In addition, nitrite ions may be produced and react with any secondary and tertiary amines present forming nitrosamines, which
are potentially carcinogenic.
C) Aldehydes
A number of aldehydes
possess broad-spectrum antimicrobial properties, including sporicidal activity. These highly effective biocides can be employed in appropriate conditions as
chemosterilants.
i) Glutaraldehyde
Glutaraldehyde (CHO(CH2)3CHO) has a broad spectrum of antimicrobial activity and rapid
rate of kill,
most vegetative bacteria being
killed within a minute of exposure, although bacterial spores may require 3 hours or more.
The kill rate
depends on the intrinsic resistance of spores, which may vary widely.
It has the further advantage of not being
affected significantly by organic matter.
The glutaraldehyde molecule possesses two aldehyde groupings which are highly reactive
and their presence
is an important component of biocidal activity. The monomeric molecule is in equilibrium with polymeric forms, and the physical conditions of temperature and
pH have a significant effect on this equilibrium. At a pH of 8,
biocidal activity
is greatest but stability is poor due to
polymerization. In contrast, acid solutions are stable but considerably less active, although as
temperature is increased, there is a breakdown in the polymeric forms which exist in acid solutions
and a concomitant increase in free, active
dialdehyde, resulting in better activity. In practice, glutaraldehyde is generally supplied as an acidic 2% or greater
aqueous solution, which
is stable on prolonged storage. This
is then ‘activated’ before use by addition of a suitable alkalizing agent to bring
the pH of the solution to its optimum for
activity. The activated solution will have
a limited shelf
life, of the
order of 2 weeks, although more stable formulations are
available. Glutaraldehyde is employed mainly
for the cold liquid
chemical sterilization of medical and surgical materials that cannot be sterilized by
other methods. Endoscopes, including for example
arthroscopes, laparascopes, cystoscopes and bronchoscopes, may be decontaminated by glutaraldehyde treatment (see section 2.5
concerning toxicity issues). Times employed in practice for high-level
disinfection are often considerably less than the many
hours
recommended by manufacturers to achieve sterilization. The contact
time for sterilization can be as long
as 10 hours. Times for general disinfection generally range from
20–90 minutes at 20 °C depending on formulation and concentration.
ii)
Ortho-phthalaldehyde
Ortho-phthalaldehyde (OPA) is a
relatively recent addition to the aldehyde
group of high-level disinfectants. This agent has demonstrated excellent mycobactericidal activity with complete kill of M. tuberculosis within 12 minutes
at room temperature. OPA has several other advantages over glutaraldehyde. It requires no activation, is considerably less irritant to the eyes or nasal passages and has
excellent stability over
the pH range
3–9. It can
be used for
disinfection of endoscopes (Table 19.5).
iii) Formaldehyde
Formaldehyde (HCHO) can be used in either the liquid
or the gaseous state
for disinfection purposes. In the vapour phase it has been used for decontamination of isolators, safety cabinets
and rooms. The combination of formaldehyde vapour with low-temperature steam (LTSF) has been employed
for the sterilization of heatsensitive items . Formaldehyde vapour is highly toxic and potentially carcinogenic if
inhaled, thus its use must be carefully controlled. It is not very active at temperatures below
20 °C and requires a relative
humidity of at least
70%. The agent
is not supplied as a gas but either
as a solid polymer, paraformaldehyde, or a liquid, formalin, which is a 34–38% aqueous
solution. The gas is liberated by heating or mixing the solid or liquid with potassium permanganate and water. Formalin, diluted 1 : 10 to give 4% formaldehyde, may be used for
disinfecting surfaces. In general, however, solutions of either aqueous
or alcoholic formaldehyde are too irritant for routine application to skin, while poor penetration and a tendency to polymerize on surfaces limit
its use as a disinfectant for pharmaceutical purposes.
iv) Formaldehyde-releasing agents
Various
formaldehyde
condensates have been developed to reduce the irritancy associated with formaldehyde while maintaining activity, and these
are described as formaldehyde-releasing agents or masked-formaldehyde
compounds.
Noxythiolin (N-hydroxy N-methylthiourea) is supplied as a dry powder and on aqueous
reconstitution slowly releases formaldehyde and N-methylthiourea. The compound has extensive antibacterial and antifungal properties and has been used both topically
and in accessible body cavities
as an irrigation solution and in the treatment of peritonitis. Polynoxylin (poly[methylenedi(hydroxymethyl)urea]) is a similar compound available in gel and lozenge
formulations. Taurolidine (bis-(1,1-dioxoperhydro-1,2,4-thiadiazinyl-4) methane) is a condensate of two molecules of the amino acid taurine and three
molecules of formaldehyde. It is more stable
than noxythiolin in solution and has similar uses.
D) Biguanides
i) Chlorhexidine
Chlorhexidine is an antimicrobial agent first synthesized in 1954. The chlorhexidine molecule, a bisbiguanide, is symmetrical with a hexamethylene
chain linking two biguanide groups,
each with a para-chlorophenyl radical (Figure 19.3).
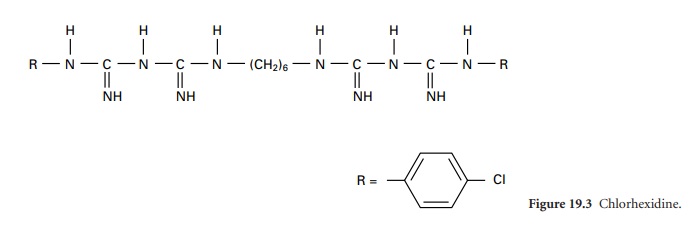
Chlorhexidine base is
not readily soluble in water; therefore its freely
soluble salts, acetate, gluconate and
hydrochloride, are used in formulation. Chlorhexidine exhibits the greatest
antibacterial activity at pH 7–8 where it exists exclusively as a dication. The
cationic nature of the compound
results in activity
being reduced by anionic
compounds, including soap, due to the formation of insoluble salts. Anions to be wary of include
bicarbonate, borate,
carbonate, chloride, citrate
and phosphate, with avoidance of hard water
if possible. Deionized
or distilled water should preferably be used for dilution purposes. Reduction in activity will also occur
in the presence of blood, pus and other
organic matter.
Chlorhexidine has widespread use, in particular as an antiseptic. It has significant antibacterial activity, although Gram-negative bacteria are less sensitive
than Gram-positive organisms. A concentration of 0.0005% prevents
growth of, for example, Staph. aureus, whereas 0.002% prevents growth of Ps. aeruginosa. Reports
of pseudomonad contamination of aqueous chlorhexidine solutions have prompted
the
inclusion of small amounts of ethanol or isopropanol.
Chlorhexidine is ineffective at ambient
temperatures against bacterial spores and M. tuberculosis. Limited antifungal activity has been demonstrated, which unfortunately restricts
its use as a general
preservative. Skin sensitivity has occasionally been reported although, in general, chlorhexidine is well tolerated and non-toxic when applied
to skin or mucous membranes and is an important preoperative antiseptic.
ii) Polyhexamethylene Biguanides
The antimicrobial
activity of the bisbiguanide chlorhexidine exceeds that of monomeric
biguanides. This stimulated the development of polymeric biguanides containing repeating biguanide groups linked by hexamethylene chains. One such compound is a commercially
available heterodisperse mixture of polyhexamethylene biguanides (PHMB, polyhexanide) having the general formula
shown in Figure 19.4.
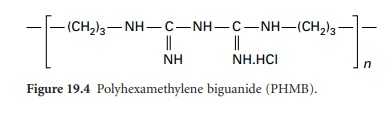
Within the structure, n varies with a mean value of 5.5.
The compound
has a broad spectrum of activity against Gram-positive and Gram-negative bacteria and has low toxicity. PHMB
is employed as an antimicrobial agent in various ophthalmic products.
E) Halogens
Chlorine and iodine
have been used extensively since their introduction as disinfecting agents
in the early 19th century. Preparations containing
these halogens, such as Dakin’s solution and
tincture of iodine,
were early inclusions in many pharmacopoeias and national formularies. More recent formulations of these elements have improved activity, stability and ease of use.
i) Chlorine
A large number of
antimicrobially active chlorine compounds are commercially available, one of the most important being liquid chlorine. This is supplied
as an amber liquid
made by compressing and cooling gaseous chlorine. The terms liquid
and gaseous chlorine refer to elemental chlorine, whereas the word
‘chlorine’ itself is normally used to signify a mixture of OCl−, Cl2, HOCl and other active chlorine compounds
in aqueous solution.
The potency of chlorine disinfectants is usually expressed in terms of parts per million (ppm)
or percentage of available chlorine (avCl).
ii)
Hypochlorites
Hypochlorites (bleach)
are the oldest
and remain the most useful of the chlorine
disinfectants, being readily available, inexpensive and compatible with most anionic and cationic surface-active agents. They exhibit
a rapid kill against
a wide spectrum of microorganisms, including fungi and viruses. High levels of available chlorine will enable eradication of
mycobacteria and bacterial spores. Their disadvantages are that they
are corrosive, suffer inactivation by organic matter and can become unstable. Hypochlorites are
available as powders or liquids, most frequently as the sodium or potassium
salts of
hypochlorous acid (HOCl). Sodium hypochlorite exists in solution as follows:
NaOCl + H2O = HOCl + NaOH (1)
Undissociated hypochlorous acid is a strong
oxidizing agent and its potent antimicrobial activity is dependent on pH as shown:
HOCl = H+ + OCl (2)
At low pH the existence of HOCl is favoured over OCl− (hypochlorite ion). The relative microbicidal effectiveness of these
forms is of the order
of 100 : 1. By lowering
the pH of hypochlorite solutions the antimicrobial
activity increases to an optimum
at about pH 5. However
this is concurrent with a decrease
in stability of the solutions. This problem
may be alleviated by addition
of NaOH (see equation 1) in order
to maintain a high pH during storage for stability. The absence of buffer allows the pH to be lowered sufficiently for activity on dilution
to use-strength. It is
preferable to prepare use-dilutions of hypochlorite on a daily
basis.
Undiluted bleach
stored at room
temperature in a closed
container has a shelf life of about
6 months. Storage of stock or working
solutions of bleach
in open containers causes
release of chlorine
gas, especially at elevated temperatures, and this considerably weakens the antimicrobial
activity of the
solution. Working
solutions should be prepared on a daily basis.
iii) Organic chlorine Compounds
A number
of organic chlorine,
or chloramine, compounds are now available for disinfection and antisepsis.
These are the N-chloro (=N-Cl) derivatives of, for example, sulphonamides giving compounds such as chloramine-T and dichloramine-T, and halazone
(Figure 19.5), which
may be used
for the disinfection of contaminated drinking-water.
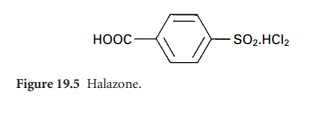
A second group of
compounds, formed by N-chloro derivatization of heterocyclic compounds containing a
nitrogen in the ring, includes the sodium and potassium
salts of dichloroisocyanuric acid (e.g.
NaDCC). These are available in granule
or tablet form and, in contrast to hypochlorite,
are very stable
on storage if protected from moisture. In water they will give a known
chlorine coccentration. The
antimicrobial activity of the compounds is similar to that
of the hypochlorites when acidic
conditions of use are maintained. It is, however, important to note that
where inadequate ventilation exists, care must
be taken
not to apply
the compound to acidic fluids
or large spills
of urine in view of the toxic
effects of chlorine production. The HSE has
set the occupational exposure standard (OES) short-term exposure limit at 1 ppm.
iv)
Chloroform
Chloroform
(trichloromethane, CHCl3) has a narrow spectrum of activity. It has
been used extensively as a preservative of pharmaceuticals since the 19th century, although more recently it has had limitations placed
on its use. Marked
reductions in concentration may occur through
volatilization from products, resulting in the possibility of microbial growth.
v)
Iodine
Iodine has a wide
spectrum of antimicrobial activity. Gram-negative and Gram-positive organisms, bacterial spores (on extended
exposure), mycobacteria, fungi and viruses are all susceptible. The active agent is the elemental iodine molecule, I2. As elemental iodine is only
slightly soluble in water, iodide
ions are required for aqueous solutions such as Aqueous Iodine Solution, BP
1988 (Lugol’s Solution) containing
5% iodine in 10% potassium iodide solution.
Iodine (2.5%) may also be dissolved in ethanol (90%)
and potassium iodide
(2.5%) solution to give Weak Iodine
Solution, BP 1988 (Iodine
Tincture).
The antimicrobial activity of iodine
is less dependent than chlorine on temperature and pH, although
alkaline pH should be avoided.
Iodine is also less susceptible to inactivation by organic matter.
Disadvantages in the use
of iodine in skin antisepsis are staining of skin and fabrics
coupled with possible sensitizing of skin and mucous
membranes.
vi)
Iodophors
In the 1950s iodophors
(iodo meaning iodine and phor meaning carrier) were developed, to eliminate the
disadvantages of iodine while retaining its antimicrobial activity. These allowed slow
release of iodine
on demand from the complex formed.
Essentially, four generic
compounds may be used as the carrier molecule
or complexing agent. These give polyoxymer iodophors (i.e. with
propylene or ethyene oxide
polymers), cationic (quaternary ammonium) surfactant iodophors, non-ionic
(ethoxylated) surfactant iodophors and polyvinylpyrrolidone iodophors (PVP-I
or povidone-iodine). The non-ionic or cationic surface-active agents act as solubilizers and carriers, combining detergency with antimicrobial activity.
The former type of surfactant, especially, produces a stable, efficient formulation, the activity of which is further enhanced by the addition of phosphoric or citric
acid to give a pH below
5 on use-dilution. The iodine
is present in the form of micellar
aggregates which disperse on dilution, especially below the critical micelle concentration (cmc) of the surfactant, to liberate free
iodine.
When iodine
and povidone are combined, a chemical
reaction takes place
forming a complex
between the two. Some of the iodine
becomes organically linked
to povidone, although the major portion
of the complexed iodine is in the form
of tri-iodide. Dilution
of this iodophor results in a weakening of the iodine
linkage to the carrier polymer with concomitant increases
in elemental iodine in solution
and antimicrobial activity.
The amount
of free iodine
the solution can generate is termed the ‘available iodine’. This acts as a reservoir for active iodine, releasing it when required
and therefore largely avoiding
the harmful side effects of high iodine concentration. Consequently, when used for
antisepsis, iodophors should
be allowed to remain on the skin
for 2 minutes to obtain full advantage of the sustained-release iodine.
Cadexamer-I2 is an iodophor similar to povidoneiodine. It is a 2-hydroxymethylene cross-linked (1–4) α D-glucan carboxymethyl ether containing iodine. The compound is
used especially
for its
absorbent and antiseptic properties in the management of leg ulcers and pressure sores where it is applied in the form of microbeads containing 0.9% iodine.
F) Heavy Metals
Mercury and silver have
antibacterial properties and preparations of these
metals were among
the earliest used antiseptics;
however, they
have been largely
replaced by less toxic
compounds. Silver has enjoyed a renaissance
recently as an antimicrobial frequently incorporated in
urethral catheters for the prevention of device-related
infection. Various forms of silver are employed such as
nanoparticulate silver, silver
halides, silver oxide and combinations such as silver–palladium. A hard surface disinfectant formulation based on
silver dihydrogen citrate is shown to be effective against a wide
range of bacteria, fungi and viruses using as little
as 30 ppm silver.
i) Mercurials
The organomercurial
derivatives thiomersal and phenylmercuric nitrate or acetate (PMN or PMA) (Figure
19.6) have been primarily employed
as preservatives. Use of both compounds has declined considerably as a result of
concerns about mercury
toxicity and risk of hypersensitivity or local
irritation. They are absorbed from solution by rubber
closures and plastic
containers to a significant extent.
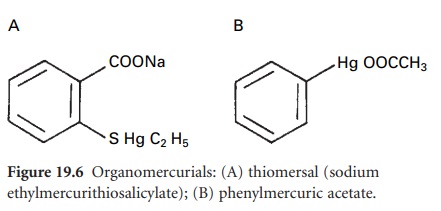
G) Hydrogen Peroxide And Peroxygen Compounds
Hydrogen peroxide
and peracetic acid
are high-level disinfectants because of their production of the highly reactive hydroxyl
radical. They have the added advantage that their decomposition products
are non-toxic and biodegradable. The germicidal properties of hydrogen
peroxide (H2O2) have been
known for more
than a century, but use of low concentrations of unstable solutions did little for its reputation. However, stabilized solutions are now available and because
of its unusual properties and antimicrobial
activity, hydrogen peroxide has a valuable
role for specific applications. Its activity against
the protozoan Acanthamoeba, which can cause
keratitis in contact
lens wearers, has made it popular for disinfection of soft
contact lenses.
Concentrations of 3–6% are effective for general disinfection purposes. At high concentrations (up to 35%)
and increased temperature, hydrogen peroxide
is sporicidal. Use has
been made of this in vapour-phase
hydrogen peroxide decontamination of equipment and enclosed spaces.
Peracetic acid (CH3COOOH) is the peroxide
of acetic acid and is a more potent
biocide than hydrogen peroxide, with excellent rapid biocidal activity
against bacteria, including mycobacteria, fungi, viruses and spores. It can
be used in both the liquid and vapour phases and is active
in the
presence of organic
matter. It is finding
increasing use at concentrations of 0.2–0.35% as a chemosterilant of medical equipment such as flexible
endoscopes. Its disadvantages are that it is corrosive to some metals.
It is also highly irritant and must be used in an enclosed system. The combination of hydrogen peroxide and peracetic acid is synergistic and
is marketed as a cold
sterilant for
dialysis machines.
H) Phenols
Phenols (Figure
19.7) are widely
used as disinfectants and preservatives. They have good antimicrobial activity and are rapidly bactericidal but generally are not sporicidal. Their activity is markedly diminished by dilution and is also reduced
by organic matter.
They are more
active at acid pH. Major disadvantages include
their caustic effect on skin and tissues and their systemic
toxicity. The more
highly substituted phenols are less toxic and can be used as preservatives and antiseptics; however, they are also less active than the simple phenolics, especially against Gram-negative organisms. To improve
their poor aqueous solubility, phenolic disinfectants are often formulated with soaps, synthetic detergents, and/or solvents.
i) Phenol (carbolic acid)
Phenol (Figure
19.7A) no longer
plays any significant role as an antibacterial agent. It is largely of historical interest, as it was used by Lister
in the 1860s as a surgical antiseptic and has been
a standard for comparison with
other disinfectants
in tests such
as the Rideal–Walker test.
ii) Clear Soluble Fluids, black Fluids and white Fluids
Phenols obtained by distillation of coal or petroleum can be
separated by fractional distillation according to their
boiling point range into phenols,
cresols, xylenols and high boiling point
tar acids. As the boiling
point increases bactericidal activity increases and tissue
toxicity decreases, but there
is increased inactivation by organic matter
and decreased
water solubility.
Clear soluble
fluids are produced from cresols or xylenols. The preparation known
as Lysol (Cresol
and Soap Solution BP 1968) is a soap-solubilized formulation of cresol (Figure 19.7B)
that has been widely used as a general-purpose disinfectant but has largely been superseded by less irritant phenolics. A higher boiling
point fraction consisting of xylenols and
ethylphenols (Figure 19.7C and D) produces a more active,
less corrosive product that retains
activity in the presence of organic
matter. A variety of proprietary products for general disinfection purposes are available.
Black fluids
and white fluids
are prepared by solubilizing the high boiling point
tar acids. Black
fluids are homogeneous
solutions that form
an emulsion on dilution with water, whereas
white fluids are finely dispersed stable emulsions. Both types of fluid have
good bactericidal activity. Preparations are very irritant
and corrosive to skin; however, they are relatively inexpensive and are useful for household and general disinfection purposes.
iii)
Synthetic phenols
Many derivatives
of phenol are now made
by a synthetic process. A combination of alkyl or aryl substitution and halogenation of phenolic compounds
has produced useful derivatives. Two of the best known chlorinated derivatives are p-chloro-m-cresol (chlorocresol, Figure 19.7E) which was frequently employed as a
preservative at a concentration of 0.1%, and p-chloro-m-xylenol (chloroxylenol, Figure 19.7F) which is sometimes used for
skin disinfection. Chloroxylenol is sparingly soluble in water and must
be solubilized, for example, in a suitable soap solution in conjunction with
terpineol or pine oil. Its antimicrobial capacity
is weak and is reduced
by the presence of organic matter. Other
phenol derivatives of note
are: 2-benzyl-4-chlorophenol (Figure 19.7G), 2-phenylphenol (Figure
19.7H) and p-tert-amylphenol
(Figure 19.7I).
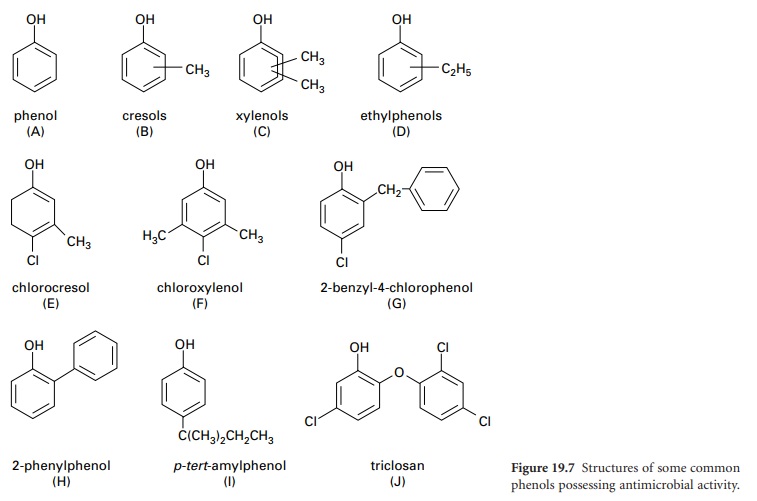
iv)
Bisphenols
Bisphenols are composed of two phenolic
groups connected by various
linkages. Triclosan (Figure
19.7J) is the most widely used. It has been incorporated into medicated soaps, lotions
and solutions and is also included in household products such as plastics and fabrics. There is
some concern
about bacterial resistance developing to triclosan.
I) Surface-Active Agents
Surface-active agents
or surfactants are classified as
anionic, cationic, non-ionic or ampholytic according to the ionization of the hydrophilic group in the molecule. A hydrophobic, water-repellent
group is also present. Within the various
classes a range
of detergent and disinfectant
activity is found. The anionic and non-ionic surface-active agents, for example, have strong detergent properties but exhibit
little or no antimicrobial activity. They can, however, render certain bacterial species
more sensitive to some antimicrobial agents,
possibly by altering the permeability of the outer envelope. Ampholytic or amphoteric agents
can ionize to give anionic, cationic and zwitterionic (positively and negatively charged
ions in the same molecule) activity. Consequently, they
display both the detergent properties of the anionic
surfaceactive agents
and the antimicrobial activity of the cationic
agents. They are used quite extensively
in Europe for presurgical
hand-scrubbing, medical instrument disinfection and floor disinfection in hospitals.
Of the four classes
of surface-active agents
the cationic compounds play the most important role in an antimicrobial context.
i) Cationic Surface -active agents
The cationic agents used for
their antimicrobial activity all fall within the
group known as the quaternary ammonium compounds (QACs, quats or onium ions). These are organically substituted
ammonium compounds (Figure 19.8A) where
the R substituents are alkyl
or heterocyclic radicals to give compounds such as benzalkonium chloride (Figure19.8B), cetyltrimethylammonium
bromide (cetrimide) (Figure 19.8C) and cetyl-pyridinium
chloride (Figure 19.8D).
Inspection of the structures of these
compounds (Figure 19.8B
and C) indicates that a chain
length in the range C8–C18 in at least
one of the R substituents is
a requirement for good antimicrobial activity.
In the pyridinium compounds (Figure
19.8D), three of the four covalent
links may be satisfied by the
nitrogen in a pyridine
ring. Several ‘generational’ changes have arisen in the development of QACs.
Compounds such as alkyldimethylbenzyl ammonium
chloride, alkyl-dimethyl-ethyl-benzyl ammonium chloride and dodecyl-dimethyl-ammonium
chloride have roles in disinfection
where HIV and HBV are present. Polymeric quaternary ammonium salts such
as polyquaternium 1 are finding increasing use as preservatives.
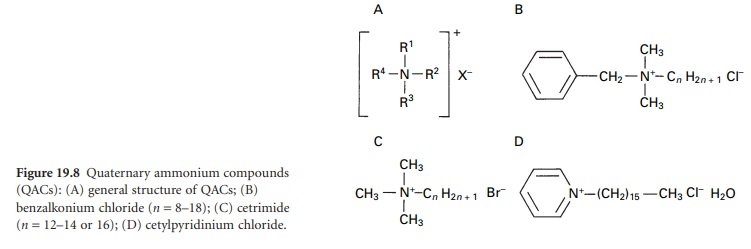
The QACs are
most effective against
microorganisms at neutral or slightly alkaline
pH and become virtually
inactive below pH 3.5. Not surprisingly, anionic
agents greatly
reduce the activity of these compounds. Incompatibilities have also been recorded with non-ionic
agents, possibly
due to the formation of micelles. The presence of organic
matter such as serum, faeces
and milk will also seriously
affect activity.
QACs exhibit greatest activity
against Gram-positive bacteria, with a lethal effect
observed using concentrations as low as 0.0005%. Gram-negative bacteria are
more resistant, requiring a level of 0.0033%, or higher still
if Ps. aeruginosa is present. A limited antifungal activity is exhibited and they have no useful sporicidal activity. This
relatively narrow spectrum
of activity limits
the usefulness of the compounds, but as they are generally
well tolerated and non-toxic when applied to skin and mucous membranes they have considerable use in treatment of wounds and abrasions. Benzalkonium chloride and cetrimide are employed extensively in surgery, urology and gynaecology as aqueous and alcoholic solutions and as creams. In many
instances they are used
in conjunction with a biguanide disinfectant such as chlorhexidine. The detergent properties of the QACs also provide
a useful activity, especially in hospitals, for general environmental cleaning.
J) Other Antimicrobials
The full
range of chemicals that can be shown to have
antimicrobial properties is beyond the scope of this
chapter. The agents included
in this section
have limited use or are of historic
interest.
i) Diamidines
The activity
of diamidines is reduced by acid pH and in the presence of blood and serum. Propamidine and dibromopropamidine, as the isethionate salts, have been
employed as antimicrobial agents
in eye drops (0.1%) for amoebic infection and
for topical treatment of minor
infections.
ii)
Dyes
Crystal violet
(Gentian violet), brilliant green and malachite green are triphenylmethane dyes used to stain bacteria for microscopic examination. They have a
static activity but are
no longer applied
topically for the
treatment of infections because
of carcinogenicity.
The acridine dyes
acriflavine and aminacrine have been employed for skin disinfection and
treatment of infected wounds or burns but
are slow acting
and mainly bacteriostatic.
iii)
Quinoline derivatives
The quinoline
derivatives of pharmaceutical interest are little used now. The compound most frequently used is dequalinium
chloride, a bisquaternary ammonium derivative of 4-aminoquinaldinium which
was formulated as a
lozenge for the
treatment of oropharyngeal infections.
K) Antimicrobial Combinations And Systems
There is no ideal
disinfectant, antiseptic or preservative. All chemical agents
have their limitations in terms of either their antimicrobial activity, resistance to organic
matter, stability,
incompatibility, irritancy, toxicity
or corrosivity. To overcome the limitations of an individual agent, formulations consisting of combinations of agents
are available. For example, ethanol
and isopropanol have been combined with chlorhexidine, QACs, sodium hypochlorite
and iodine to produce more
active preparations. The combination of chlorhexidine and cetrimide
is also considered to improve
activity. QACs and phenols have been combined with
glutaraldehyde and formaldehyde so that the same effect
can be achieved
with lower, less irritant concentrations of the aldehydes. Some combinations are considered to be synergistic, e.g. hydrogen
peroxide and peroxygen compounds. Care must be taken
in deciding on disinfectant combinations, as the concentration exponents associated with each component of a disinfectant combination will have a considerable effect on the degree of activity
.
Research into the resistance of microbial biofilms
provides potential for improving elimination of this problematical microbial mode of growth. Bacteria
often use a communication system, quorum sensing
(QS), to regulate virulence factor production and
the formation of biofilms. Increased
understanding of how chemicals can block QS could help provide effective
prevention and elimination of biofilm-related infection. The incorporation of antimicrobial agents into materials that form working and contact
surfaces or those
of medical devices and implants has been positive but much further
developmental
research is required. Such ‘bioactive’ surfaces can be formed,
for example, by incorporation of silver
salts and alloys, biguanides and
triclosan, and have
the ability to reduce
infection arising from
microbial adherence and biofilm formation.
Other means
are available to potentiate the activity of disinfectants. Ultrasonic energy in
combination with suitable disinfectants such as aldehydes and biguanides has been demonstrated to be useful
in practice and ultraviolet radiation increases the activity of hydrogen peroxide. Super-oxidized water provides
an extremely active disinfectant
with a mixture of oxidizing
species produced from the electrolysis of saline. The main
products are hypochlorous
acid (144 mg/L)
and free chlorine
radicals. The antimicrobial activity is rapid against a wide range of microorganisms in the absence
of organic matter.
Related Topics
