Mechanisms of Resistance to Biocides
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Non-Antibiotic Antimicrobial Agents: Mode Of Action And Resistance
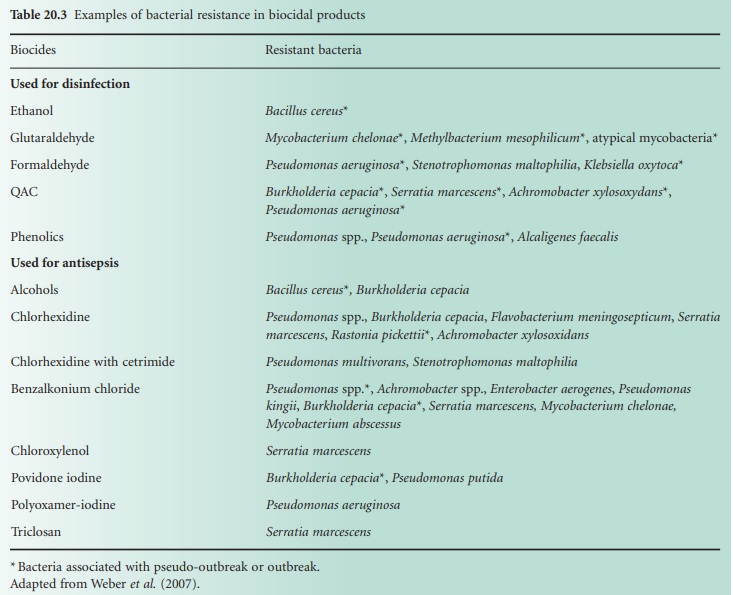
Bacterial resistance to biocides has been reported since the 1950s, notably with QACs, biguanides and phenolics. Overall there has been more documented evidence of bacterial resistance to antiseptics than to disinfectants.
MECHANISMS OF RESISTANCE TO BIOCIDES
Bacterial resistance to biocides has
been reported since the 1950s, notably with QACs, biguanides and phenolics.
Overall there has been more documented evidence of bacterial resistance to
antiseptics than to disinfectants (Table 20.3).
It is worth mentioning that some bacteria surviving in biocidal formulations
have been associated with outbreaks and pseudo-outbreaks of infection (Weber et al., 2007). Bacterial resistance to all known
preservatives has also been reported (Chapman et al., 1998).
Recently, much interest has focused on bacteria surviving highlevel
disinfection, which is usually employed for the disinfection of medical devices.
Thus bacteria surviving exposure to the in-use (high) concentration of highly
reactive biocides (e.g. glutaraldehyde, chlorine dioxide, hydrogen peroxide)
have been isolated and studied. In 2009, a large outbreak of atypical
mycobacteria in at least 38 hospitals in Brazil was reported. These
mycobacteria were traced to endoscope contamination and were resistant to 2%
w/v glutaraldehyde but also to the clinical concentration of front-line
antibiotics against mycobacteria (Duarte et al., 2009). This
was the first time that biocide resistance was linked to antibiotic resistance,
nosocomial infection and a large infection outbreak.
Over the
last 10 years,
much progress has
been made in understanding the mechanisms conferring
resistance to biocides in bacteria. Interestingly, some mechanisms that were thought to occur only with antibiotics have now been described with biocides.
a) General Mechanisms
A number
of mechanisms conferring some level of resistance to biocide exposure have been documented. Traditionally
mechanisms of resistance have been divided into intrinsic and acquired
resistance. Intrinsic
(or innate) resistance
is a natural property of the bacteria
and provides some
explanation as to why some
bacteria are less susceptible than others. Intrinsic
mechanisms often involve a structural difference, for example, a difference
in the permeability of the bacterial membrane to biocides, but also
the expression of chromosomal genes
such as those encoding for an efflux
pump or a degradative enzyme. Acquired
resistance refers to the acquisition of a new property by the bacteria
through mutation and genetic transfer; such
a property can be the mutation of a target site or the transfer of a gene encoding for an
efflux pump
or a degradative enzyme. It should be noted
that when gene transfer
occurs, often several
genes present on the same conjugative plasmid
or transposon can be transferred to a recipient cell at the same time.
In this case the term co-resistance is often used to denote
the simultaneous acquisition of a number
of genes conferring resistance to a number of antimicrobials, both
biocides and antibiotics.
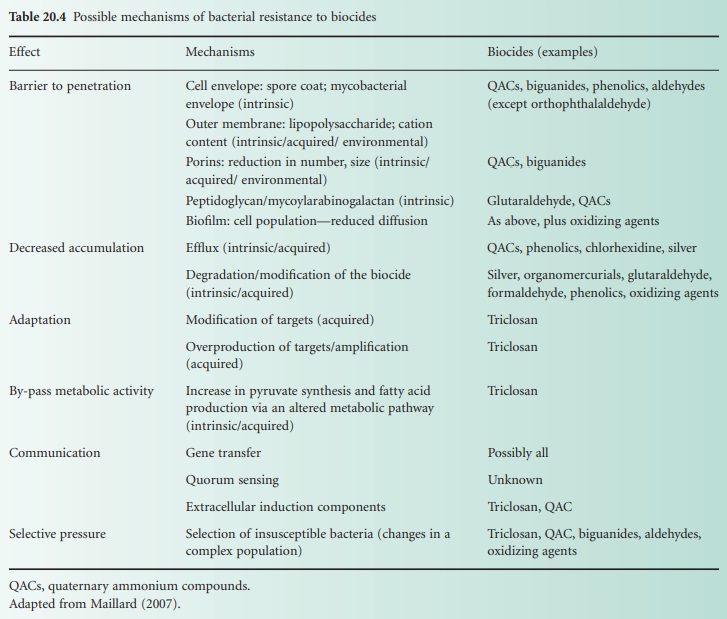
Bacterial resistance to a biocide
arises often from
the presence of several
mechanisms that work together to decrease the detrimental concentration of the biocide
to a level that
is no longer harmful for the bacterium (Table 20.4). The
expression of only one mechanism confers low-level resistance often measured as
an increase in minimum inhibitory
concentration (MIC), but rarely high-level resistance, which can be measured as an
increase in minimum
bactericidal concentration. Finally, a
distinction can be made between
mechanisms expressed by a single
bacterium and the mechanisms of resistance
that arise from a community of bacteria such
as in bacterial biofilms.
i) Changes in cell permeability
The decrease
in biocide penetration arising from changes in cell permeability is well established and has been described with different bacterial genera, notably with Gram-negative bacteria and mycobacteria. It is also
the case with bacterial endospores, which are discussed
later in this chapter. In
Gram-negative bacteria, the outer membrane,
and notably the composition of LPS, offers some protection to the
cell, by reducing biocide penetration. The role of LPS has been exemplified by researchers with the use of permeabilizing agents
and notably ion chelators such as EDTA. EDTA contributes to the removal (loss) of LPS
from the outer
membrane by scavenging dications involved
in the stabilization of LPS in the membrane (section 4.4.1).
By losing LPS, the outer membrane
becomes more permeable and biocides can
penetrate better resulting in enhanced activity. A change in the structure of the outer membrane following
a change in protein, fatty acid or phospholipid composition has been associated with a decrease in the efficacy
of cationic biocides. In particular, a decrease in the number of porin proteins as a result
of biocide exposure
has been associated with high-level resistance to QACs in pseudomonads. Recently, a change
in surface charge in Pseudomonas
aeruginosa has been
associated with a decrease in susceptibility to QACs.
In mycobacteria, the lipid-rich outer
cell wall (responsible for the waxy
appearance of the colonies), and particularly the presence of a mycoyl-acyl-arabinogalactan layer and the composition of the
arabinogalactan/ arabinomannan within the cell wall, account
for a reduction in biocide
penetration; increasing the permeability
of the mycobacterial outer
cell wall, for example with ethambutol, enhances the activity
of biocides and antibiotics.
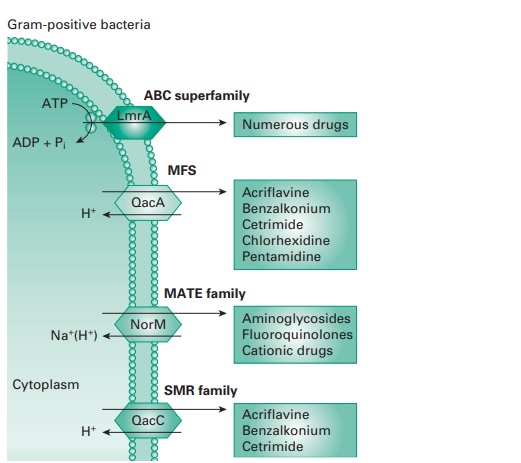
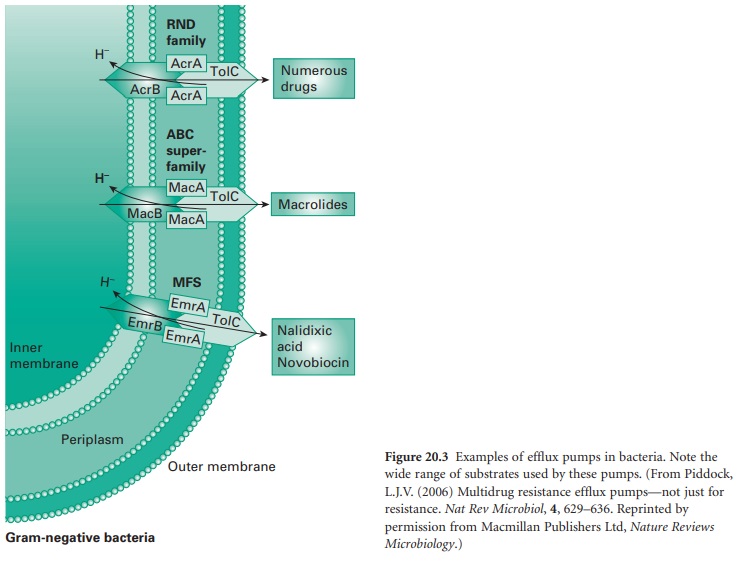
ii) Efflux
Efflux pumps are cross-membrane proteins which pump out
various substrates including biocides and antibiotics. A large number
of efflux pumps
in bacteria have been
identified and have been divided
into five main classes
depending on their structure and activity: the small
multidrug resistance (SMR) family; the major facilitator superfamily (MFS); the ATP-binding cassette (ABC) family; the resistance-nodulation-division
(RND) family; and the
multidrug and toxic compound extrusion (MATE)
family (Figure 20.3).
The role
of efflux pumps
is to remove harmful substances from the bacterial cytoplasm, including biocides (e.g. QACs and phenolics), to levels that
are not damaging
for the cell. The quantity
of antimicrobial pumped out depends upon the number of pumps present,
their expression and efficacy. Some studies have shown that high-level resistance can be achieved by
efflux, for instance against
the bisphenol triclosan, but usually,
the effect is an increase
in MIC.
iii) Enzymatic inactivation
Enzymatic degradation plays a role in reducing
the harmful concentration of a biocide
and has been
observed with aldehydes (e.g. aldehyde dehydrogenase), oxidizing agents (e.g.
catalases, superoxide dismutase, hydroxyperoxidases), phenolics and parabens. In the case of metallic salts such as silver,
the ionic form
is reduced to the inactive metal. The
role of enzymatic inactivation has not been widely studied, but it is unlikely that a bacterial enzyme will contribute to high-level resistance to a biocide.
iv)
Modification of target site
To date, resistance conferred by the modification of a biocide target site has only been observed
with triclosan. At a low concentration this bisphenol interacts
specifically with a bacterial
enoyl-acyl reductase carrier
protein, which is involved in the synthesis of fatty acid.
Triclosan has been shown to interact with a number
of structurally related enzymes
in many bacterial
genera. A modification of the enzyme
confers a low-level resistance to triclosan, although some studies
claim that a high-level resistance has been observed. This is unlikely, since at a high concentration triclosan interacts
with multiple target
sites to bring about a bactericidal effect.
v)
Change in metabolic pathway
A change in metabolic pathway that confers
resistance to a biocide is a relatively new concept that
was thought to occur only with sulphonamides. However, bacterial adaptation to triclosan, as measured by an extended
lag phase of growth followed by a normal exponential phase,
has been observed in several bacterial genera. The recent use of a microarray in Salmonella
enterica serovar Typhimurium enabled the identification of a ‘triclosan resistance network’ including an alternative
pathway to the production of pyruvate and fatty acid.
In Staphylococcus aureus showing reduced
sensitivity to triclosan, a change in lipid composition of the cell
membrane was associated with altered expression of various genes
involved in fatty
acid metabolism. Low-level QAC resistance in Serratia marcescens may arise from a change
in synthetic or metabolic pathways.
b) Induction Of Resistance
The induction of antimicrobial resistance in bacteria following biocide exposure is a
relatively recent concern which is particularly pertinent to the increasing number of commercially available
products containing a low concentration of a biocide. This low, often subinhibitory, concentration can induce the expression of resistance
mechanisms that can confer
bacterial survival to biocide
and/or antibiotic exposure. Low-level resistance as measured by an increase in MIC has often been observed. Prior to
the use of genomics, proteomics and metabolomics,
induction of resistance in bacteria was observed with an increase in lag phase
of growth and a decrease
in growth rate, an overexpression of efflux pumps
and the production of guanosine 5′-diphosphate 3′-diphosphate
(ppGpp). An increase in DNA repair
was also associated
with an increase
in bacterial survival
following biocide exposure.
More recently, a change in expression of regulons commanding a number
of responses, such
as a stress type of response (and repair mechanism), increased
efflux, change in membrane composition, and a change in
metabolic and synthetic pathways, has been recorded
following exposure
to a low concentration of biocides.
Such a global response
is of concern as it also confers
a decreased susceptibility to antibiotics, and recent evidence
suggests it might also lead to overexpression of virulence determinants.
It can be noted
that the concentration of a biocide that promotes mutation in bacteria might
be low. Indeed, a number of investigations have observed that the mutation rate increases in the presence
of an active efflux system. The effect of bacterial mutation
in the development of resistance has not been widely investigated, with the exception of triclosan. It is also
possible that biocides that interact with the bacterial genome (e.g. dyes, oxidizing
agents) might produce
a higher mutation rate.
c) Dissemination Of Resistance
Surprisingly, the
dissemination of biocide resistance mechanisms
between bacteria has been little
studied. The acquisition of new genetic determinants, notably by the process
of conjugation, is of concern
as it is often
dependent on the presence of large transferable
plasmids, and transposons which encode for many
genes including bacterial resistance to antibiotics and virulence factors. When several
resistance genes are transferred at the same time,
the term co-resistance is used. For example,
the resistance to the QAC benzalkonium chloride in Staphylococcus aureus has been associated with the presence of plasmids containing qac, bla and tet resistant genes (encoding for efflux pumps
and a β-lactamase). A number of
mechanisms of resistance such as efflux
and degradative enzymes
have been documented to be transferred between
bacteria. The extent
of such dissemination is difficult
to measure, although
it is thought to readily
occur in bacterial communities such as a biofilm.
Equally important to the horizontal transfer of resistance is the maintenance of resistant determinants (plasmids) following the continuous presence of biocides. Although this
issue has not been widely
studied, it is of
interest with the use of biocidal
products which are documented to leave a residual
concentration of biocide
on surfaces, antimicrobial surfaces, and the continuous presence of biocides in certain applications, such as drinking water chlorination.
d) Bacterial Spores
The formation of a spore
is a mechanism of bacterial survival when growth conditions are detrimental
for the vegetative form. Such adverse
conditions include lack of food but also the presence of biocides and other detrimental physical and chemical
conditions. The spore structure is unique and confers upon
the spore high level resistance to biocides. Hence only a few biocides, mainly highly reactive
ones such as aldehydes, oxidizing agents and chlorine-releasing agents,
are sporicidal, while others such as biguanides, QACs and phenolics are sporostatic
despite their bactericidal effect on the vegetative bacteria. It should also
be noted that
sporicides take time to kill spores, usually
a minimum of 5 minutes contact, and at a high concentration; aldehydes
such as glutaraldehyde and formaldehyde need a much longer
contact time and, in the case of ortho-phthalaldehyde, a raised temperature.
i) Sporulation and germination
Sporulation, a process in which a bacterial spore
develops from a
vegetative cell , involves seven stages (I–VII); of these, stages
IV–VII (cortex and coat
development) are the most important in relation to the development of biocide resistance. Resistance to biocidal agents develops during sporulation and
may be an early, intermediate or late/very late
event. For example, resistance to chlorhexidine
occurs at an intermediate stage, at about the same time as heat resistance, whereas decreasing susceptibility to glutaraldehyde is a very
late event.
Bacillus spore coatless mutants and chemically induced coatless spores have shown
the role of the spore coats in limiting access
of biocides to the spore
core. The cortex also acts as a barrier to some extent.
During germination and/or outgrowth, metabolism and biosynthetic processes increase and cells regain their sensitivity to antibacterial
agents. Some inhibitors act at the germination stage (e.g. phenolics, parabens), whereas others such as chlorhexidine and the QACs do not affect germination but inhibit outgrowth. Glutaraldehyde, at low concentrations, is an effective
inhibitor of both stages.
ii) Spore Structure
The spore structure and the interaction between a biocide
and the spore
have been particularly well documented in the genus Bacillus. The spore core (protoplast,
sometimes referred to as the germ cell)
is enclosed within a cell wall which is surrounded by the cortex and several
spore coats. Sometimes an exosporium may surround the spore.
The spore
core is the target site
of sporicides since
it is the location of RNA, DNA, dipicolinic acid (DPA) and most of the calcium, potassium, manganese and phosphorus. Also present are substantial
amounts of low molecular
weight basic proteins, the small acid-soluble spore proteins (SASPs) which are rapidly degraded during germination. SASPs,
comprising about 10–20%
of the protein in the dormant spore, exist in two forms (α/β and γ) and are essential for expression of spore resistance to ultraviolet radiation and also appear
to be involved in resistance to some biocides
e.g. hydrogen peroxide. Spores (α−/β−) deficient in α/β-type SASPs are much more peroxide-sensitive than are wild-type (normal)
spores. It has been proposed that in wild-type
spores DNA is saturated with
α/β-type SASPs and is thus
protected from free radical
damage.
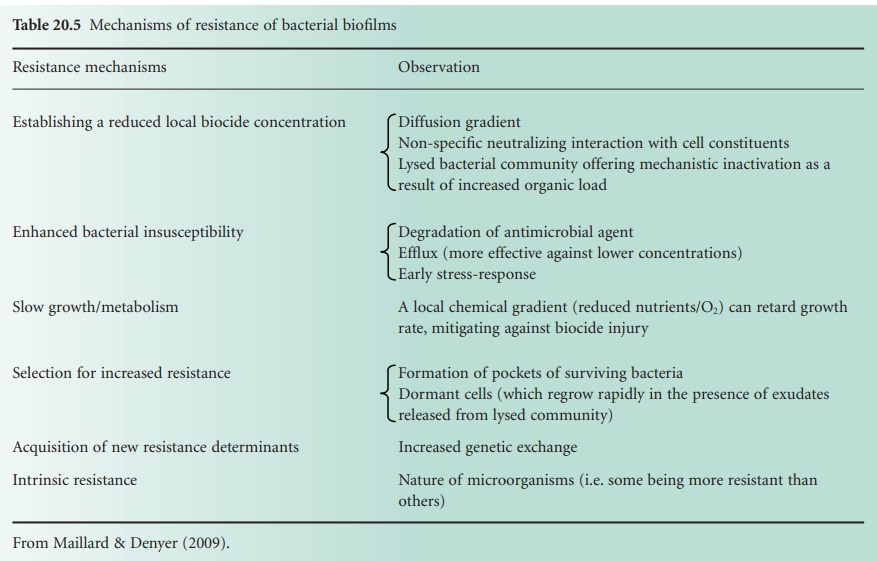
e) Bacterial Biofilms
Bacteria are generally associated with surfaces in a
complex community called biofilms. Following attachment to a surface, a bacterium will go through a series of metabolic and phenotypic changes, leading to the formation of microcolonies embedded within a matrix of secreted exopolysaccharides. Bacteria in biofilms have been shown
to be less susceptible to antibiotics and biocides than
planktonic bacteria. There are several
biocide resistance mechanisms, all contributing
to a ‘biofilm-associated phenotype’: reduction in biocide penetration, reduced
bacterial metabolism, quiescence, enzymatic
inactivation and efflux (Table 20.5).
Biocides have been observed
to change the composition of a complex
biofilm, composed of different bacterial genera or/and species. For example, polyhexamethylene biguanides (PHMB), chlorhexidine and Bardac (a QAC) have
been shown to select for pseudmonads to the detriment of Gram-positive bacteria. The bisphenol
triclosan was shown
to reduce the genera diversity of a complex waste drain biofilm and
to decrease the
overall susceptibility of the remaining population.
f) Misuse And Abuse Of Biocides
The indiscriminate use of biocides
in an increasing number of applications and, notably,
the use of sub-optimal
low concentrations has fuelled the debate on emerging bacterial cross-resistance to antibiotics used for human and animal
medicine. This is based on in vitro
evidence that some mechanisms conferring a decrease
in biocide susceptibility can also lead to resistance to therapeutic concentrations of antibiotics. Some of the most
common mechanisms involved include
expression and
over-expression of efflux
pumps and changes
in cell permeability and metabolism. However, there is no useful
rule of thumb to predict cross-resistance between biocide and
antibiotic resistance in bacteria. In addition, emerging cross-resistance following biocide exposure in situ has
not been
widely reported. The most significant study to date was reported
in 2009 and concerned an outbreak in 38 hospitals of an isolate
of Mycobacterium massiliense resistant to 2% glutaraldehyde and to antimycobacterial therapeutic
antibiotics.
Related Topics
