Factors Influencing Drug Absorption and Bioavailability
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Absorption of Drugs
To achieve the desired therapeutic objective, the drug product must deliver the active drug at an optimal rate and amount.
FACTORS INFLUENCING DRUG ABSORPTION AND BIOAVAILABILITY
Biopharmaceutic Considerations in Dosage Form Design
To achieve the desired therapeutic objective, the drug product must deliver the active drug at an optimal rate and amount. By proper biopharmaceutic design, the rate and extent of drug absorption (also called as bioavailability) or the systemic delivery of drug to the body can be varied from rapid and complete absorption to slow and sustained absorption depending upon the desired therapeutic objective. The chain of events that occur following administration of a solid dosage form such as a tablet or a capsule until its absorption into systemic circulation are depicted in Fig. 2.14.
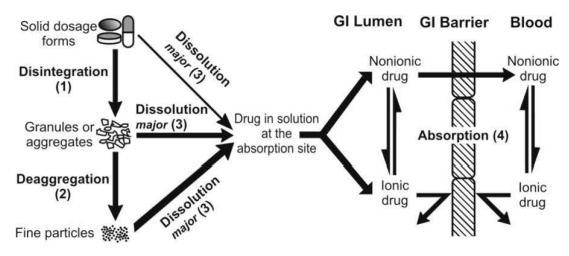
Fig. 2.14. Sequence of events in the absorption of drugs from orally administered solid dosage forms
The process consists of four steps:
1. Disintegration of the drug product.
2. Deaggregation and subsequent release of the drug.
3. Dissolution of the drug in the aqueous fluids at the absorption site.
4. Absorption i.e. movement of the dissolved drug through the GI membrane into the systemic circulation and away from the absorption site
As illustrated in Fig. 2.14, the drug may also dissolve before disintegration or deaggregation of the dosage form, and before or after reaching the absorption site. Unless the drug goes into solution, it cannot be absorbed into the systemic circulation.
In a series of kinetic or rate processes, the rate at which the drug reaches the systemic circulation is determined by the slowest of the various steps involved in the sequence. Such a step is called as the rate-determining or rate-limiting step (RDS). The rate and extent of drug absorption from its dosage form can be influenced by a number of factors in all these steps. The various factors that influence drug absorption (also called as biopharmaceutic factors in the dosage form design) can be classified as shown in Table 2.2.
TABLE 2.2.
Factors influencing GI Absorption of a Drug from its Dosage Form
A. PHARMACEUTICAL FACTORS: include factors relating to the physicochemical properties of the drug, and dosage form characteristics and pharmaceutical ingredients
I. Physicochemical Properties of Drug Substances
1. Drug solubility and dissolution rate
2. Particle size and effective surface area
3. Polymorphism and amorphism
4. Pseudopolymorphism (hydrates/solvates)
5. Salt form of the drug
6. Lipophilicity of the drug
7. pKa of the drug and gastrointestinal pH
8. Drug stability
9. Stereochemical nature of the drug
II. Dosage Form Characteristics and Pharmaceutical Ingredients (Pharmaco-technical Factors)
1. Disintegration time (tablets/capsules)
2. Dissolution time
3. Manufacturing variables
4. Pharmaceutical ingredients (excipients/adjuvants)
5. Nature and type of dosage form
6. Product age and storage conditions
B. PATIENT RELATED FACTORS: include factors relating to the anatomical, physiological and pathological characteristics of the patient
1. Age
2. Gastric emptying time
3. Intestinal transit time
4. Gastrointestinal pH
5. Disease states
6. Blood flow through the GIT
7. Gastrointestinal contents:
a. Other drugs
b. Food
c. Fluids
d. Other normal GI contents
8. Presystemic metabolism by:
a. Luminal enzymes
b. Gut wall enzymes
c. Bacterial enzymes
d. Hepatic enzymes
Related Topics
