Metabolic Reactions
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Cellular Metabolism
1. What are the functions of catabolism and anabolism? 2. Explain the differences between dehydration synthesis and hydrolysis. 3. What are the three primary stages involved in processing nutrients for energy release?
Metabolic
Reactions
Metabolism consists of the chemical changes thattake place inside
living cells. As a result of metabolism, organisms grow, maintain body
functions, release or store energy, produce and eliminate waste, digest
nutrients, or destroy toxins. These reactions alter the chemical nature of a
chemical substance, maintaining homeostasis.
Two major types of metabolic
reactions control how cells use energy. The buildup of larger mole-cules from
smaller molecules is called anabolism. An example of anabolism is when amino acids bond together and form
proteins. The breakdown of larger molecules into smaller ones is called catabolism. An
example of catabolism is when foods are hydrolyzed in the digestive tract. Both
anabolism and catabolism require the use of energy.
Anabolism
Anabolism is the process of
building complex molecules in the body from simpler materials. When a person
is healthy and has adequate nutrition, simple nutrients (such as amino acids,
fats, and glucose) are used by the body to build the basic chemicals that
sup-port cellular functioning and sustain life.
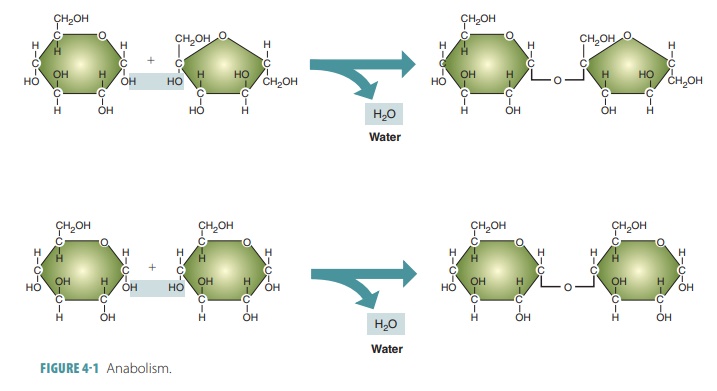
Anabolism supplies biochemicals
needed for cells to grow and repair themselves. An example of anab-olism is
when simple sugar molecules called mono-saccharides
are linked to form a chain, making upmolecules of glycogen (a
carbohydrate). This anabolic process is called dehydration
synthesis. As the links in this chain are
formed, an OH (hydroxyl group) is removed from one molecule, whereas an H
(hydrogen atom) from another is removed. Together, the OH and H produce a water
molecule (H2O). The monosaccha-rides are then joined by a shared
oxygen atom, making the chain grow (FIGURE 4-1).
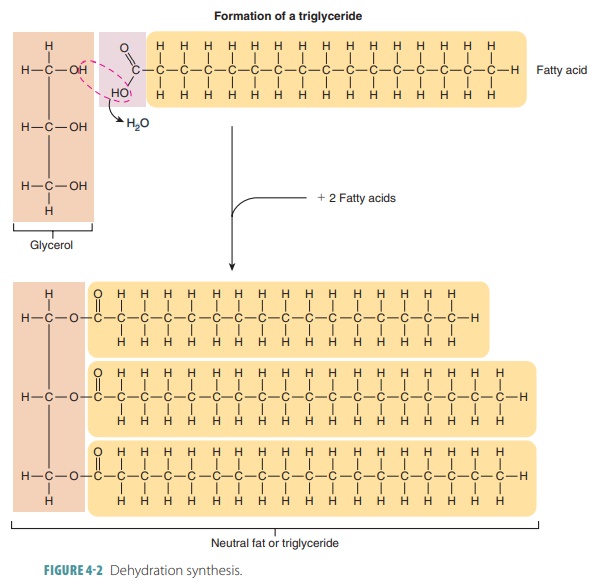
Dehydration synthesis, which
links glycerol and fatty acid molecules in adipose (fat) cells, forms fat
molecules (triglycerides). This occurs when three hydrogen atoms are removed
from a glycerol molecule. An OH group is removed from each of three fatty acid
molecules (FIGURE 4-2 ). This creates three water molecules and one fat molecule. Oxygen
atoms are then shared between the glycerol and fatty acid portions.
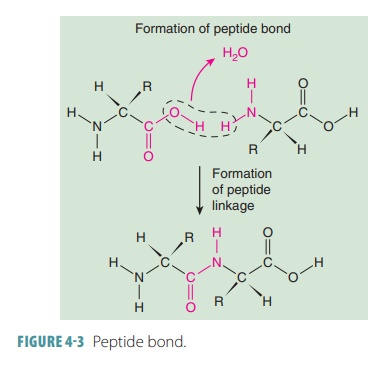
Cells also use dehydration
synthesis to join amino acid molecules, eventually forming protein molecules.
As two amino acids unite, one OH mol-ecule is removed from one of them, whereas
one H molecule is removed from the NH2 group of another. This forms
a water molecule. The amino acid mole-cules are then joined by a bond created
between a nitrogen atom and a carbon atom, called a peptidebond (FIGURE 4-3).
A dipeptide is formed from two amino acids bound together and a polypeptide is formed from many amino
acids bound into a chain. Polypeptides usu-ally have specialized functions.
When a polypeptidehas more than 100 molecules, it is considered to be a
protein. Certain protein molecules have more than one polypeptide.
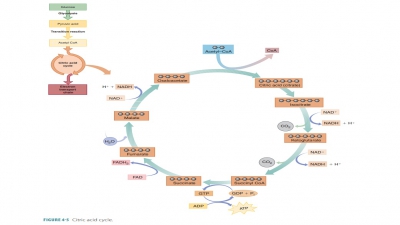
The three primary stages involved
in processing nutrients for energy release are:
■■ Stage 1: Digestion in the
gastrointestinal tract. Absorbed nutrients are transported to the tissue cells
by the blood.
■■ Stage 2: In the tissue cells,
nutrients are built into glycogen, lipids, and proteins or are broken down to
pyruvic acid and acetyl coenzyme A (CoA) in the cell cytoplasm.
■■ Stage 3: In the mitochondria,
there is much catabolic activity, requiring oxygen. This finalizes food
breakdown, produces water and carbondioxide, and collects large amounts of
adenosine triphosphate (ATP). Carbohydrates such as glucose combine with oxygen
to produce large amounts of ATP.The glycolysis occurring in Stage 2 and all
events in Stage 3 make up cellular respiration, which is discussed in detail
later in this chapter.
Catabolism
Catabolism can be defined as the
metabolic breakdown of stored carbohydrates, fats, or proteins to provide
energy. It occurs continuously to differing degrees. Excessive catabolism leads
to wasting of tissues. An example of catabolism is the process of hydrolysis, which
is actually the opposite of dehydration synthe-sis. This involves the
decomposition of carbohydrates, lipids, and proteins.
Hydrolysis splits a water
molecule; for example, hydrolysis of sucrose (a disaccharide) gives off glucose
and fructose (two monosaccharides) as the water mol-ecule splits. The equation
is:
C12H22O11+H2O →
C6H12O4+C6H12O6
(Sucrose) (Water) (Glucose) (Fructose)
As shown in the equation, inside
the sucrose mol-ecule the bond between the simple sugars breaks. The water
molecule supplies a hydrogen atom to one of the sugar molecules while supplying
a hydroxyl group to the other.
Both dehydration synthesis and
hydrolysis are reversible and are summarized in the following equation:
Hydrolysis → Disaccharide + Water ↔ Monosaccharide + Monosaccharide ← Dehydration synthesis
During digestion, hydrolysis
breaks down carbo-hydrates into monosaccharides. It also breaks down fats into
glycerol and fatty acids, nucleic acids into nucleotides, and proteins into
amino acids.
1. What
are the functions of catabolism and anabolism?
2. Explain
the differences between dehydration synthesis and hydrolysis.
3. What
are the three primary stages involved in processing nutrients for energy
release?
Related Topics