SAR of Sulphonylureas
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Oral Hypogylcaemic Drugs
The benzene ring should contain a substitutent preferably in the para position. The substituents, such as methyl, acetyl, amino, chloro, bromo, trifluoro methyl, and dithiomethyl were found to enhance the antihyperglycaemic activity.
SAR of Sulphonylureas
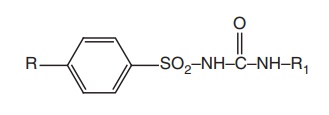
1.
The benzene
ring should contain a substitutent preferably in the para position. The
substituents, such as methyl, acetyl, amino, chloro, bromo, trifluoro methyl,
and dithiomethyl were found to enhance the antihyperglycaemic activity.
2.
When the
para position of benzene is substituted with aryl carboxamidoalkyl group
(second-generation sulphonylureas, such as glibenclamide) the activity was
found to be enhanced further.
3.
The size of
the group attached to the terminal nitrogen is crucial for activity. The group
should also impart lipophilicity to the compound N-methyl and ethyl substituents that show no activity, whereas N-propyl and higher homologues were
found to be active and the activity is lost when the N-substituent contains 12 or more carbons.
Related Topics
