Sulphonylurea derivatives
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Oral Hypogylcaemic Drugs
Sulphonylurea derivatives : a. First-generation drugs i. Tolbutamide (Orinase) ii. Chloropropamide (Diabenese) iii. Tolazamide (Tolamide, Tolinase) iv. Acetohexamide b. Second-generation drugs i. Glibenclamide (Glyburide) ii. Glipizide (Dibizide, Glucolip, Glynase) iii. Gliclazide
SYNTHESIS AND DRUG PROFILE
Sulphonylurea derivatives
a. First-generation drugs
Mode of action: They target on the specific receptors in the β
cells of islets of Langerhans called sulphonylurea receptors, and cause
depolarization by reducing the conductance of ATP sensitive K+ channels. This
enhances the Ca2+ influx and produces degranulation, leading to the secretion of
insulin.
The
sulphonylureas may be represented by the following general structure
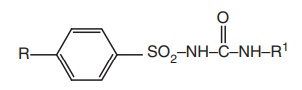
All members
of this group are urea derivatives with an aryl sulphonyl group in the 1st
position and an aliphatic group at the 3rd position. The R group on the
aromatic ring primarily influences the duration of action of the compound.
i. Tolbutamide (Orinase)
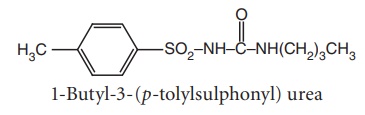
Properties and uses: Tolbutamide is a white crystalline powder,
practically insoluble in water, soluble in acetone, alcohol, and dilute
solutions of alkali hydroxides. It is useful in the treatment of selected cases
of diabetes mellitus, namely, mild and uncomplicated, stable diabetes of adult.
Assay: Dissolve the sample in a mixture of water and alcohol (1:2) and
titrate against 0.1 M sodium hydroxide using phenolphthalein as indicator.
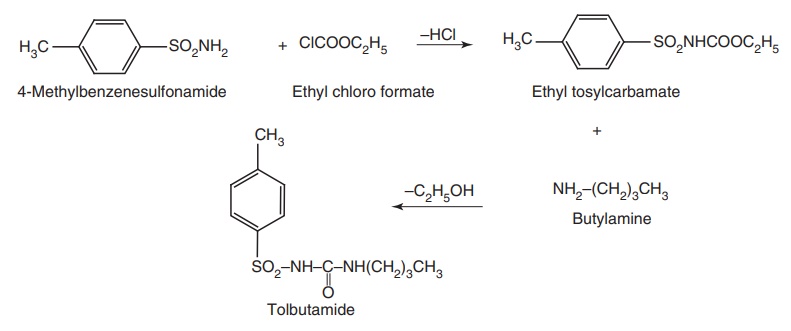
Synthesis
Route I. From: 4-Methylbenzene sulphonamide
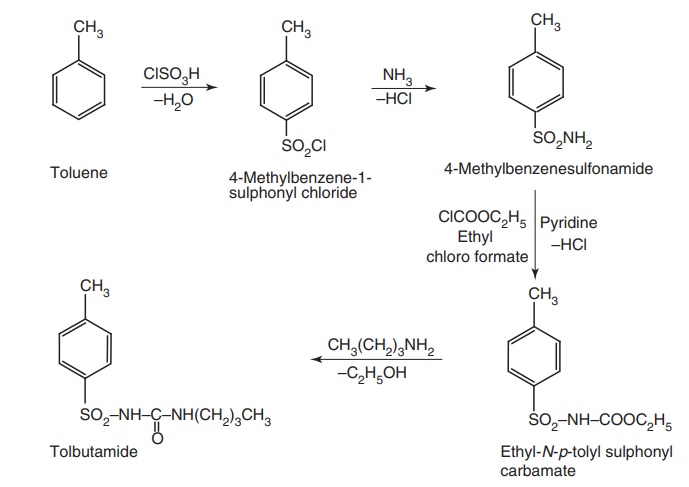
Route II. From: Toluene
Dose: The usual dosage is 250 and 500 mg with an initial dose of 500
mg.
Dosage forms: Tolbutamide tablets B.P.
ii. Chloropropamide (Diabenese)
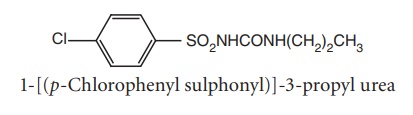
Synthesis
Route I. From: 4-Chlorobenzenesulphonamide
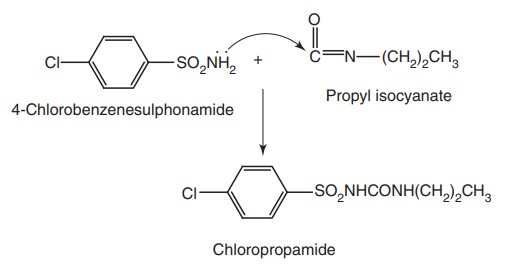
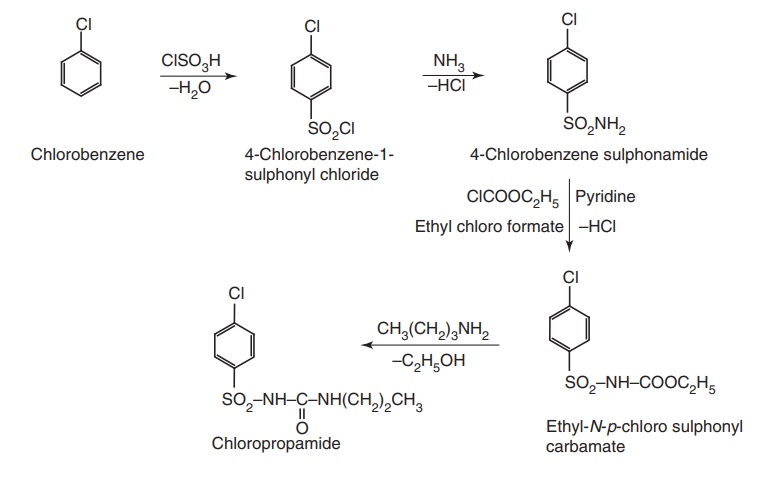
Ruote II. From: Chlorobenzene
Metabolism: It is metabolized by ω or ω-1 hydroxylation of the propyl group.
This reaction is slow and significant amount of the drug is excreted unchanged
in urine.
Properties and uses: Chloropropamide is a white crystalline powder,
practically insoluble in water, soluble in acetone, methylene chloride,
alcohol, and dilute solutions of alkali hydroxides. Used in the treatment of
diabetes mellitus.
Assay: Dissolve the sample in alcohol and add water. Titrate against
0.1 M sodium hydroxide using phenolphthalein as indicator until a pink colour
is obtained.
Dose: The usual dose is 100–250 mg per day.
iii. Tolazamide (Tolamide, Tolinase)
Metabolism of tolbutamide and tolazamide: They undergo a more rapid benzylic oxidation,
leading to an inactive benzoic acid derivative. An alternative hydroxylation of
the aliphatic ring of tolazamide becomes active and results in a metabolite of
prolonged duration of action.
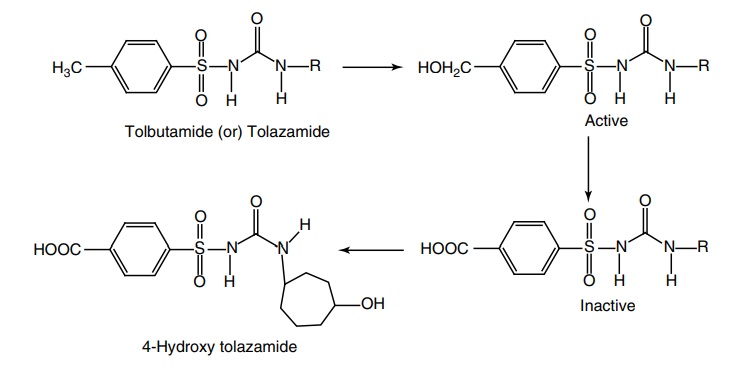
Synthesisp
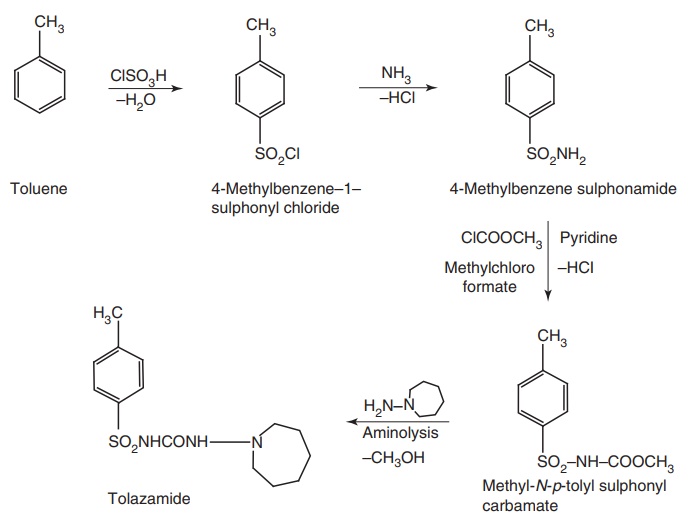
Properties and uses: Tolazmide is a white crystalline powder, very
slightly soluble in water, soluble in chloroform and acetone, slightly soluble
in ethanol. Used as an oral hypoglycaemic agent with action and uses similar to
Tolbutamide.
Assay: Dissolve the sample in butan-2-one with the aid of gentle heat. Allow to cool, add 30 ml of ethanol and titrate with 0.1 M sodium hydroxide using phenolphthalein as indicator.
Dose: The dose is 100–250 mg daily.
Dosage forms: Tolazamide tablets B.P.
iv. Acetohexamide
Synthesis
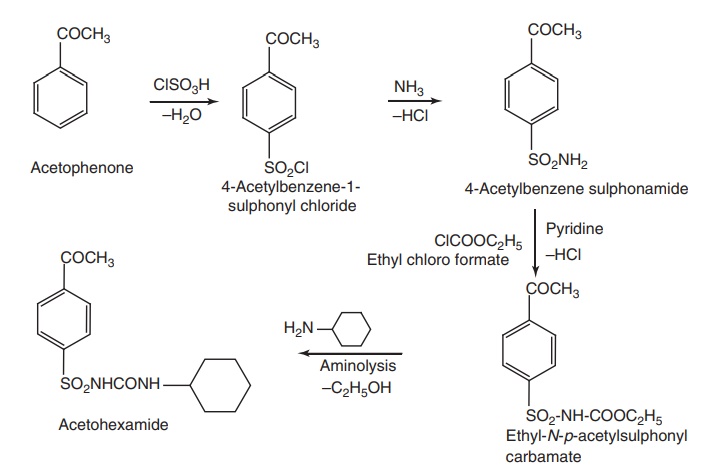
Metabolism: The major metabolite of acetohexamide is a reduced product of
the keto group, forming an alcohol. The hydroxy metabolite exhibits 2.5 times
the hypoglycaemic activity of the parent molecule.
Properties and uses: It is a white, odourless crystalline powder,
soluble in alcohol and chloroform, but insoluble in water or ether. It is an
orally active hypoglycaemic drug.
b. Second-generation drugs
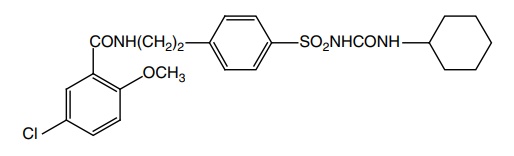
Synthesis
Route I. From: 5-Chloro-2-methoxybenzoylchloride
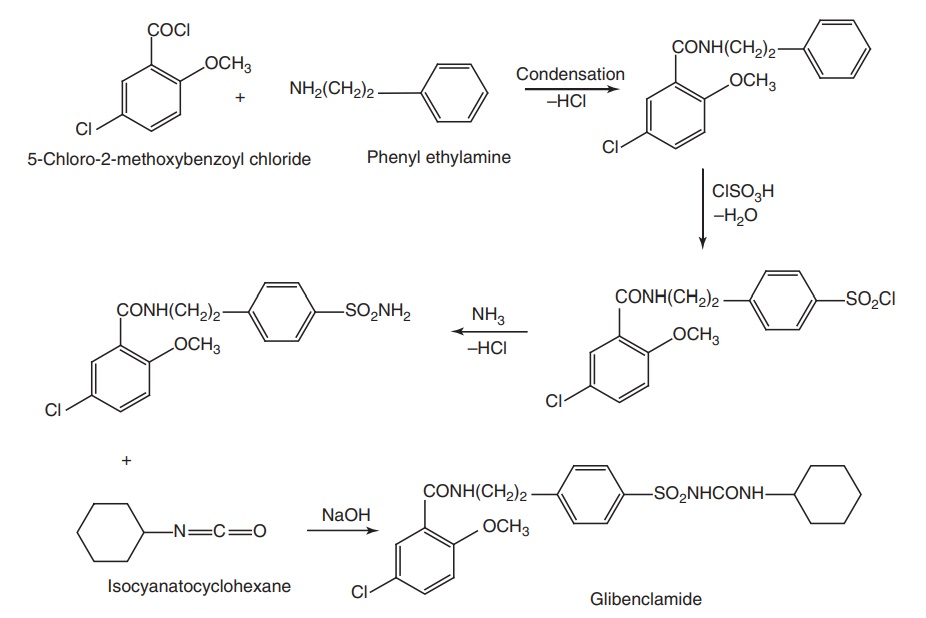
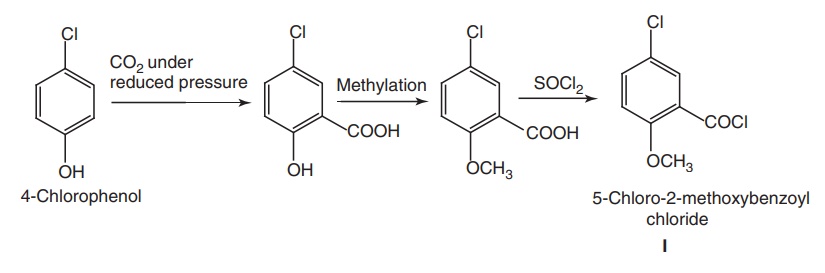
Route II.
Step I: Synthesis of
5-chloro-2-methoxybenzoyl chloride
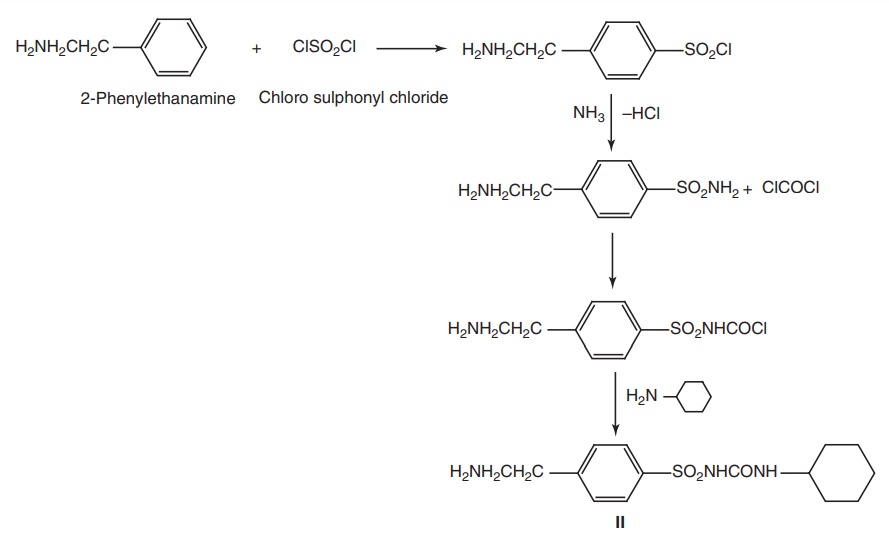
Step II: Synthesis of
4-(Aminoethyl)-1-cyclohexylamino carbonyl benzene sulphonamide
Step III: Condensation
of product of Step I and Step II
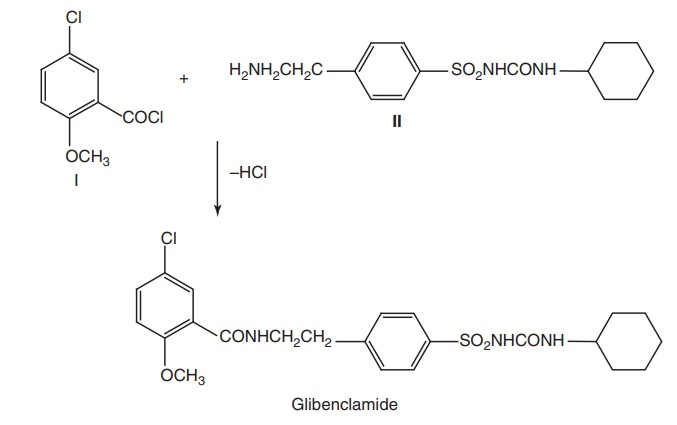
Properties and uses: Glibenclamide is a white crystalline powder,
practically insoluble in water, sparingly soluble in methylene chloride,
slightly soluble in alcohol and methanol. Used in the treatment of mild
uncomplicated NIDDM unresponsive to diet alone.
Assay: Dissolve the sample in alcohol by heating and titrate against
0.1 M sodium hydroxide, using phenolphthalein as indicator, until a pink colour
is obtained.
Dose: The dose is 2.5–5 mg per day to be taken with breakfast.
Dosage forms: Glibenclamide tablets B.P.
ii. Glipizide (Dibizide, Glucolip, Glynase)
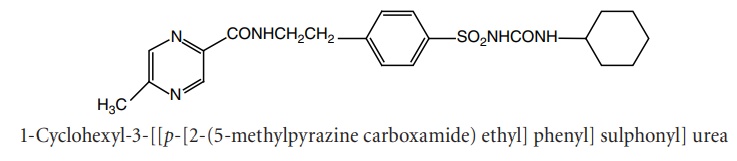
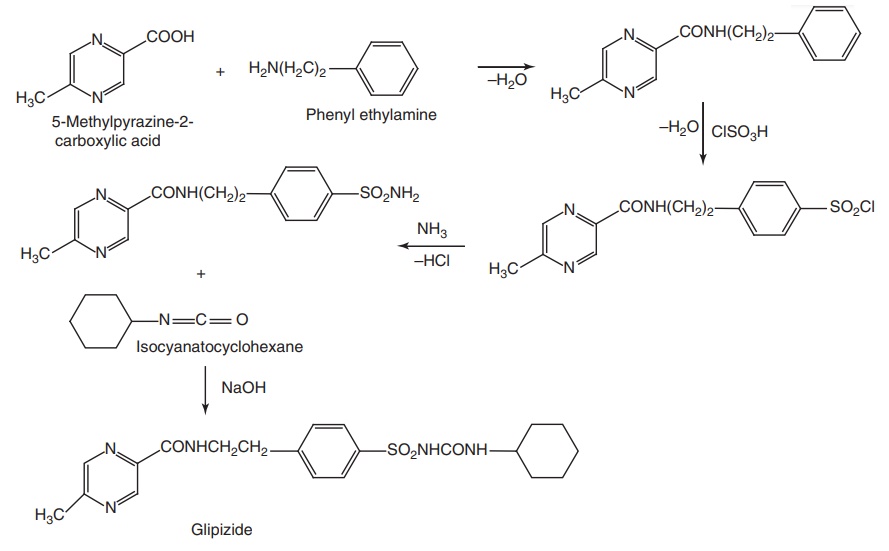
Synthesis
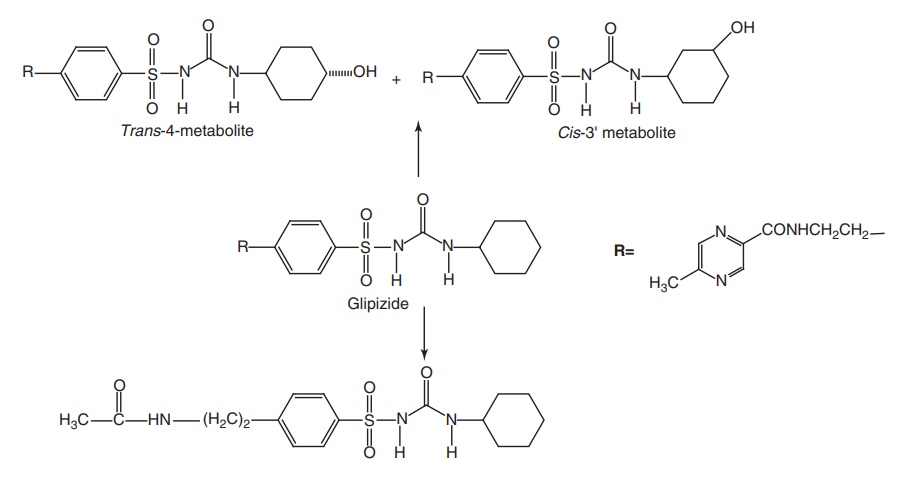
Metabolism: It is extensively metabolized to less active or inactive
metabolites. Its metabolites are excreted primarily in the urine.
Properties and uses: Glipizide is a white crystalline powder,
practically insoluble in water and ethanol, very slightly soluble in methylene
chloride and acetone, soluble in dilute solutions of alkali hydroxides. It is
an orally active hypoglycaemic drug.
Assay: Dissolve the sample in dimethylformamide and titrate against 0.1
M lithium methoxide add quinaldine red as indicator, until the colour changes
from red to colourless.
Dose: The dosage is 25–50 mg once a day or in divided doses.
Dosage forms: Glipizide tablets B.P.
iii. Gliclazide
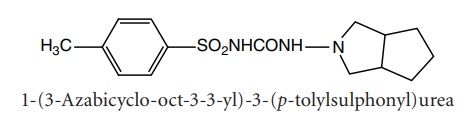
Properties and uses: Gliclazide is a white powder, practically
insoluble in water, soluble in methylene chloride, sparingly soluble in
acetone, and slightly soluble in alcohol. It is an orally active hypoglycaemic
drug.
Assay: Dissolve the sample in anhydrous acetic acid and titrate against
0.1 M perchloric acid. Determine the end point potentiometrically.
Dosage forms: Gliclazide tablets B.P.
Related Topics
