Surface area - Analyses of powders
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Powders and granules
Surface-dependent physicochemical phenomena are of pharmaceutical significance.
Surface area -
Analyses of powders
Significance of surface area
Surface-dependent
physicochemical phenomena are of pharmaceutical significance. For example,
·
Absorption of a drug from a dosage form involves dissolution
of the drug substance into the absorption medium. The rate of dissolution is
proportional to the surface area of the drug substance.
·
Lubricants, such as magnesium stearate, used during
pharmaceutical processing are intended to cover the surface of the granules to
provide adequate lubricity during unit operations such as tableting. Changes in
the surface area of the granules or the lubricant can directly impact the
surface coverage and effectiveness of the lubricant.
·
Wet granulation is a surface phenomenon, involving wetting
and agglomeration of particles. Changes in the surface area of the raw
materials can significantly influence the reproducibility of granulation.
Defining surface area
Total
surface area available in a powder sample is a function of both its particle
size and porosity. Particle size is relatively easier to measure and compare
among different powders. Porosity of the particles refers to air-filled solvent
accessible channels inside particles. Thus, porosity contributes to the surface
area of the particles without impacting particle size or shape. A higher
porosity particle of the same size and shape as a lower porosity particle will
have greater surface area. The rate of disintegration and drug dissolution from
granules depends on the penetration of the dissolution medium inside the
granules, which is determined by the porosity of the granules.
In
determination of total surface area of a powder sample, it is difficult to
distinguish the area contributed by the surface of the granules from the area
contribution attributable to the porosity. For all practical purposes, this
distinction is ignored. It is assumed that the surface area accessible to the
penetrating medium is representative of the surface area relevant to the
pharmaceutical applications of the powder.
Quantitation of surface area by gas adsorption
Surface
area is commonly measured by the adsorption of an inert gas on a solid surface.
It is commonly expressed as specific surface area, which is the surface area
per unit weight of the powder.
Adsorption
of an inert gas (the adsorbate) on a solid surface (the adsor-bent) is driven
by the weak van der Waals forces of attraction. The rate and extent of
adsorption of the gas is primarily driven by the partial pressure of the gas (P). At isothermal (constant temperature)
conditions, Freundlich proposed that the mass of gas adsorbed (x) per unit mass of adsorbent (m) is given by
x/m = k * P1/n
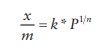
where
k and n are constants.
Freundlich
isotherm considers multiple layers of the adsorbate on the adsorbent. Langmuir
proposed an alternative equation to describe gas adsorption on the solid
surface that relies on the assumption of monolayer adsorption. The number of
sites occupied on the surface of a solid (θ) is given by
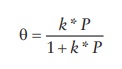
Θ = k * P / (1 + k * P)
where,
k = ka
/kd, ka and kd
representing the rate constants of adsorption and desorption processes,
respectively.
Both
Langmuir and Freundlich adsorption isotherms explain gas adsorp-tion at low
pressures, but not at high pressures. Multilayer formation dur-ing gas
adsorption was explained by the Brunauer–Emmett–Teller (BET) equation:
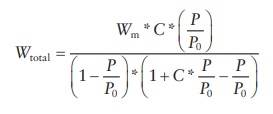
where:
P and P0 are the equilibrium and saturated vapor pressure of the adsorbate
Wtotal is the total amount of gas adsorbed
Wm is the amount of gas adsorbed to form a monolayer
C is the BET constant
that depends on the heat of adsorption for the first layer (E1),
the heat of adsorption for the second and subsequent layers or the heat of
liquefaction of the adsorbate (EL),
gas constant (R), and absolute
temperature (T) as
C = e E1−EL/RT
BET
adsorption isotherm adequately describes physical gas adsorption for θ = 0.8 to
2.0. This range covers the formation of the monolayer. The BET equation can
also be expressed as a linear equation:
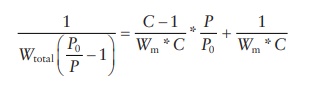
The
determination of surface area of pharmaceutical powders is most frequently
carried out using this equation. Assessment of binding interac-tions of a
dissolved drug with solid particles in solution is carried out using Langmuir
adsorption isotherm. Freundlich isotherm is used to characterize the types of
adsorption profiles of different solids.
For
the determination of powder surface area using BET method, adsorp-tion of an
inert gas, such as nitrogen, is carried out at isothermal conditions. The
number of moles of the gas adsorbed (Wtotal)
as a function of the equilibrium pressure (P)
is recorded. The use of BET equation allows the calculation of the amount of
gas that would form a monolayer (Wm),
which allows the calculation of total surface area using the molecular area of
the gas (nitrogen, 15.8 Å2) and the Avogadro’s number of molecules
per mole of substance.
Altering powder surface area
Specific
surface area of excipients and drug substances is primarily deter-mined by
their manufacturing process, which affects their PSD and poros-ity. Therefore,
making changes to their manufacturing process can change surface area of raw
materials. For example, the use of spray drying instead of slow solvent
evaporation techniques, such as drum drying, results in the production of
higher porosity particles. Changes in crystalline polymor-phic form produced as
result of crystallization process, such as the solvent used for
crystallization, can also result in changes to the specific surface area of the
material.
High-specific
surface area of APIs is often desired to increase their dis-solution rate from
the dosage forms. This is commonly achieved by com-munition or particle size
reduction. In addition, certain excipients, such as magnesium stearate, have a
unique plate-type structural
organization of the molecules, such that the application of shear and mixing
results in the separation of plates leading to increase in surface area.
Reduction
of particle surface area is desired for applications where, for example,
reduction of undesired, surface-induced phenomena is needed. For example,
sticking of the powder material to the stainless steel pro-cessing equipment
during pharmaceutical manufacture is a function of the surface characteristics
of the APIs. Therefore, reduction in the surface of the APIs per unit powder
weight can minimize or mitigate this processing risk. This is commonly achieved
by decreasing drug load in the formulation and granulation of the APIs with low
proportion of fine particles.
Related Topics
