Particle shape and size - Analyses of powders
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Powders and granules
Defining particle shape and size, Defining particle size distribution, Desired particle shape and size, Factors determining particle shape, Techniques for quantifying particle shape and size, Changing particle shape and size
Particle shape and
size - Analyses of powders
Defining particle shape and size
The
size of a sphere can be defined in terms of its radius, or more com-monly,
diameter. The size of a cube can be described in terms of the length of its
side or diagonal. However, as shown in Figure 19.1,
particles can have a diverse range of shapes from needle shape to irregular
polygo-nal. Quantitatively measuring and defining the size of these particles
can be a challenge. Nevertheless, the use of finely divided powders in
phar-maceutical unit operations requires a numerical description of particle
size, preferably as a single number, to enable comparison of different powder
types and also of different batches of the same material. Using a
one-dimensional property of a particle (such as its surface area or volume) and
describing it in terms of an equivalent sphere allow the description of a three-dimensional
object by a single number with respect to the property of interest. The
criterion of equivalency of particle size to the size of a sphere is based on
the powder’s intended use or application. For example, use of a powder for
surface catalysis or comparison of dissolution rate of different batches would
require surface area-based equivalency.
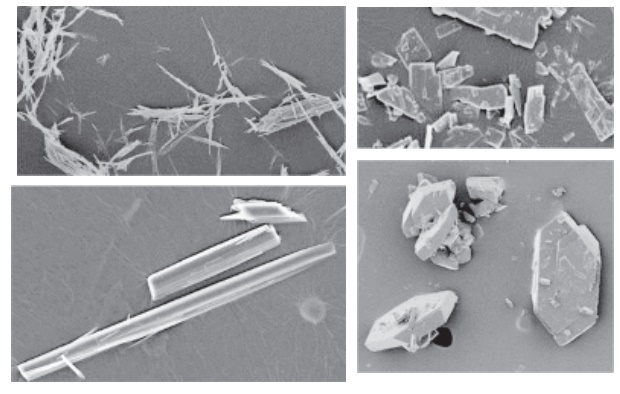
Figure 19.1 Examples of particle shapes commonly encountered for active
pharmaceutical ingredients.
Irregular-shaped
particles can be defined in terms of two parameters:
·
Diameter of an equivalent sphere. Powder processing
technologies, such as milling and granulation, tend to change the shape of
particles toward or closer to a spherical shape.
·
Aspect ratio, which is the ratio of longest to the smallest
axis of a par-ticle. It would be one for a sphere and the largest for a
needle-shaped particle. Aspect ratio helps define the deviation of a shape from
a perfect sphere.
Many
commonly used particle size measurement methods define the size of a particle
in terms of the diameter of an equivalent sphere. There are several assumptions
and/or limitations associated with this description. For exam-ple, defining
particle size in terms of the diameter of an equivalent sphere requires a consideration
of the criterion used to define equivalency.
For example, two particles can be described as equivalent in terms of volume or
surface area. Thus, size of a particle can be expressed as the diameter of a
sphere of equivalent volume or surface area of the particle being analyzed.
Defining particle size distribution
Powders
are a collection of particles of different sizes. Therefore, powders have a PSD
rather than a single particle size. A single numeric descriptor of the PSD of a
powder can be the mean particle size. The mean diameter of a set of particles
in a powder sample can be described using either arith-metic mean or geometric
mean. When using arithmetic mean diameter, the presence of fewer, larger
diameter particles can skew the calculated average result toward the large
particle size, which may not be truly representative of the batch. The
distribution of particles of a powder often follows a unimodal (one peak)
lognormal distribution (i.e., when log of particle size is plotted against the
frequency of occurrence of the particles of each size—a Gaussian or normal
distribution is obtained). Therefore, geometric mean diameter (GMD) is
generally preferred to define the particle size of a powder.
The
method for defining PSD needs to have the following properties:
·
Be independent of the statistical type of distribution in
the sample, for example, normal or lognormal.
·
Be descriptive of the particle characteristics of interest
to the intended application that is, expressing particle size as spheres of equivalent
surface area or volume depending on the application.
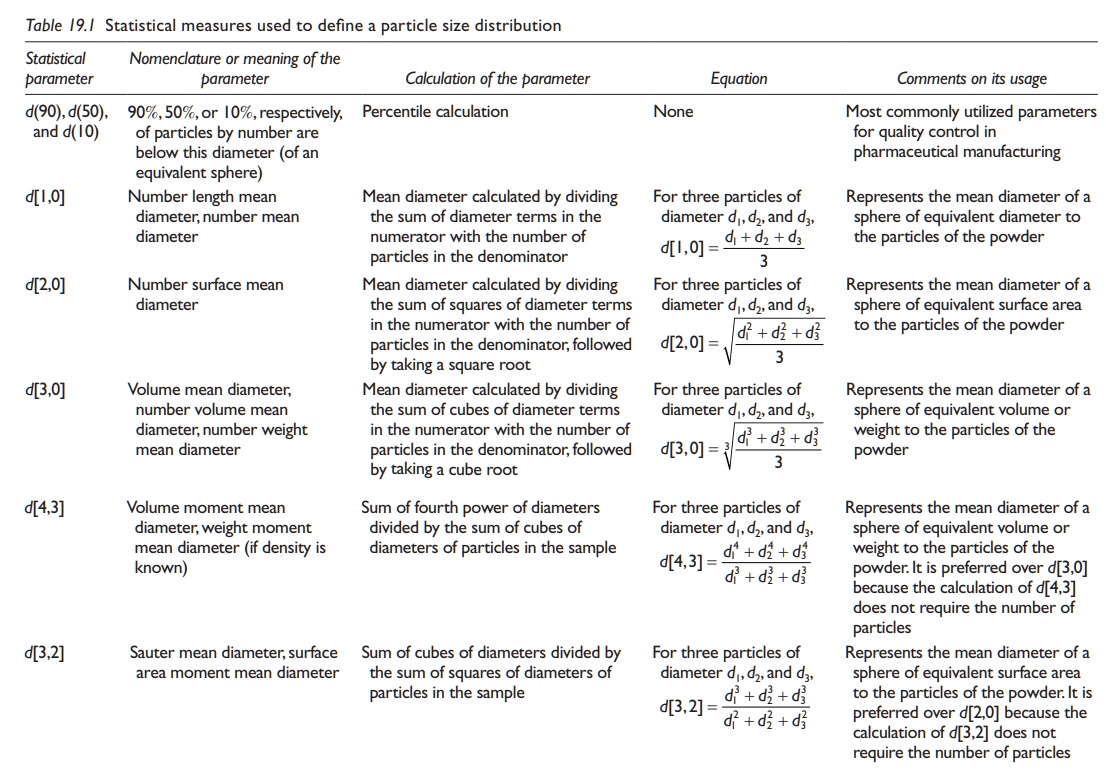
The
statistical measures listed in Table 19.1 are
frequently used to charac-terize the PSD of a powder sample.
Table 19.1 Statistical measures used to define a particle size distribution
Desired particle shape and size
The
desired particle size and shape of a powder is determined by its usage in the
downstream unit operations. For example,
·
Uniform mixing of powders is greatly facilitated if they are
of equiva-lent size by volume. Therefore, the mixing of two or more powders
with similar particle size and shape is the most likely to produce uni-form
distribution of each material in the mix.
·
Use of particles in inhalation devices requires particles to
be of simi-lar sedimentation rate in the air. Particle size expressed as
diameter of spheres with equivalent sedimentation rate in the air is called
aero-dynamic diameter.
·
The surface area per unit weight or volume (specific surface
area) of the powder determines the extent of physicochemical properties of a
material that are of surface origin. For example, for crystal pack-ing
structures that lead to the exposure of functional groups on the surface, a
polymorphic form with greater specific surface area is more likely to show
greater intensity of such surface phenomenon than another polymorphic form with
lower specific surface area. Examples of such crystal surface-dependent
physical properties include chemi-cal reactivity or surface adsorption in the
solid state and the sticking tendency of a material to the stainless steel
processing equipment dur-ing pharmaceutical manufacturing.
Notably,
the spherical shape offers the least surface area per unit volume or weight of
the material.
Factors determining particle shape
Particle
shape is primarily determined by the intrinsic properties of the material and
its manufacturing process. For example, crystal habits of a compound determine
the crystal faces exposed to the surface of the solid. Solute–solvent
interactions during crystallization determine which faces of a crystal grow
faster than others. In general, faces of the crystal that inter-act more with
the solvent grow at a slower pace than the faces that have less interaction
with the solvent. Thus, crystal shape is a function of the solvent used during
crystallization, and one can produce crystals of different shape having the
same crystalline or polymorphic form.
Particle
shape can be altered after crystallization. For example, milling of a drug
substance results in smaller, irregular-shaped crystals that are closer to the
spherical geometry. Also, pharmaceutical processes such as granula-tion,
spheronization, and spray drying can produce larger particles that are closer
to the spherical shape.
Techniques for quantifying particle shape and size
Particle
size is commonly measured using one or more of the following techniques:
·
Sieve analysis: This is a
conventional technique that involves mass
fractionation of a powder sample on a set of sieves, or wire meshes, of
defined size openings using mechanical vibration. Several sieves of increasing
size openings are placed one over another. A powder sample is loaded on the top
sieve. Mechanical vibration is applied to allow the powder to sift through as
many sieves as it would until it reaches a state where powder particles do not
move through the sieve openings any more. The amount of powder on each sieve is
weighed and expressed as the size fraction is lower than sieve open-ing
diameter above and is higher than the one below (on which the powder was
retained). Thus, sieve analysis produces a weight distribution of particles in
different sieve fractions. The sieve analy-sis data can be used to compare the
PSD of two or more samples graphically, or by using the calculated mean
particle diameter and/or the proportion of fines.
·
Laser diffraction
analysis:
Laser diffraction analysis is based on the
size dependence of scattering of incident laser light by particulates in
the sample. A powder sample is dispersed in an insoluble liquid or air and is
passed through a beam of laser light. The scattered laser light intensity is
recorded using a detector. The angle of light scattering decreases and the
intensity of scattered light increases with the increasing particle size.
Measuring the intensity of scat-tered light at a particular angle allows the
estimation of size of the particle scattering the light. Cumulative plotting of
size of all the particles in a powder sample produces a PSD of the sample.
·
Focused beam
reflectance measurement: In situ measurement
of particle or droplet size and size
distribution in dispersed systems is often carried out using focused beam
reflectance measurement (FBRM). This is an inline technique used to generate
real-time data during chemical synthesis, such as crystallization, and
phar-maceutical processing, such as granulation. A fast spinning laser beam is
focused on the sample through a quartz lens in a conical pattern. The laser
light that encounters a particle is reflected back to the lens, where a fiber
optic collects the light and passes to a detector that quantifies the
intensity. The time period between the incident and the reflected light, the
speed of the rotating lens, and the speed of laser light are used to calculate
the length of a par-ticle passing through the focus of the laser light. This is
called the chord length. Collective plot of chord length of several particles
produces a chord length distribution. Changes in the chord length distribution
during processing are used as a fingerprint of the pro-cess dynamics.
·
Microscopy: Microscopy allows
direct visual examination of powder particles.
However, it provides only a two-dimensional image of a three-dimensional
particle. Although this technique allows versatility with respect to sample
types that can be examined, the sample prepa-ration process can introduce bias
into the sample. It is a qualitative tool for most of the applications.
Automated image analysis software is frequently used when quantitation is
desired. Several commercial instruments are available that automate the process
of image collec-tion and analysis, allowing the examination of several hundreds
or thousands of particles in a sample.
·
Sedimentation: Sedimentation
involves gravity or centrifugal force-assisted separation of the dispersed
phase from the dispersion medium over time. The density difference between the
dispersed phase and the dispersion medium leads to particle separation.
Sedimentation is not a preferred method for the assessment of particle size and
size distribution. It is more commonly used for the quality assessment of
colloidal systems, such as suspensions and emulsions, functional-ity assessment
of superdisintegrants, such as croscarmellose sodium, and separation of
particles of extremely small size from the disper-sion medium.
·
Electrozone sensing: Changes in the
electrical conductance through a
small aperture with the flow of a fluid containing sus-pended particles are
used to estimate the size and number of parti-cles in the dispersion medium.
The electrical conductance changes when a particle flows through the aperture,
with the change in conductance being proportional to the size of the particle.
It is commonly used for counting biological cells and bacteria, using a coulter
counter.
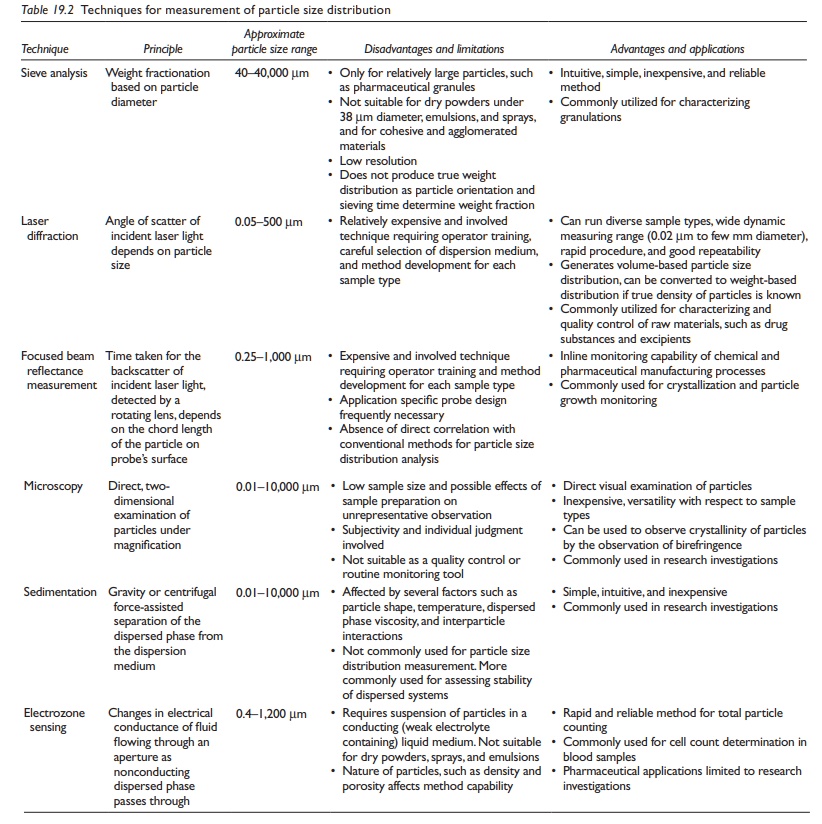
A
comparison of these techniques with respect to their merits, demerits, range of
particle size measured, and principle of operation is provided in Table 19.2.
Changing particle shape and size
Reduction
in particle size of the API is frequently desired to improve the
biopharmaceutical properties of the dosage form, such as its dissolution and
absorption. Increase in particle size of the bulk powder is generally desired
to improve its processability, such as flow properties. Particle size of the
powders can be decreased by controlled crystallization or milling (also called
comminution) of preformed particles. Particle size can be increased by
controlled agglomeration through granulation.
Table 19.2 Techniques for measurement of particle size distribution
Processing
steps to change the size of the particles invariably also results in changes in
particle shape. Milling of odd-shaped particles, such as nee-dles, tends to
reduce their aspect ratio and to change the shape toward spherical dimensions.
Granulation is often accompanied by shear force and consolidation of particles
into larger particles, which tend to have an irregular shape with low aspect
ratios. Both milling and granulation tend to increase the sphericity of
particles.
Related Topics
