Binary Mixtures of Miscible Liquids
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Evaporation and Distillation
When the two components of a binary mixture are completely miscible, the vapor pressure of a mixture is a function of mixture composition as well as the vapor pressures of the two pure components.
Binary Mixtures of Miscible
Liquids
The Relation of Vapor Pressure
and Mixture Composition
When the two components of a binary mixture are completely miscible, the vapor pressure of a mixture is a function of mixture composition as well as the vapor pressures of the two pure components. If the liquids are ideal, the relation of vapor pressure and composition is given by Raoult’s law. At a constant temperature, the partial vapor pressure of a constituent of an ideal mixture is proportional to its mole fraction in the liquid. Thus, for a mixture of A and B,
PA
= PAo
XA (10:7)
where
PA is the partial vapor pressure of A in the mixture, PAo
is the vapor pressure of pure A, and xA is its mole fraction.
Similarly,
PB
= PBoXB
(10:8)
The
total pressure of the system, P, is simply PA + PB.
These
relations can be expressed graphically. If the vapor pressure at a given
temperature of each pure component is marked on a graph of vapor pressure
versus mole fraction, the total vapor pressure at the same temperature of a
liquid mixture of any composition falls on the straight line joining the vapor
pressures of the two components. The partial pressure of each component is
indicated by the diagonals of this figure. The principle is shown in Figure
10.4. A separate relation must be constructed for each temperature.
Very
few liquid mixtures rigidly obey Raoult’s law. Consequently, the vapor pressure
data must be determined experimentally. Mixtures that deviate positively from
the law give a total vapor pressure curve that lies above the theoretical
straight line. Negative deviations fall below the line. In extreme cases,
deviations are so large that a range of mixtures will exhibit a higher or lower
vapor pressure than that of either of the pure components.
Returning
to ideal systems, the partial pressure of a component in the vapor is
proportional to its mole fraction. For component A,
PA
= yAP (10:9)
where
PA is the partial pressure of A in the vapor and yA is
its mole fraction.
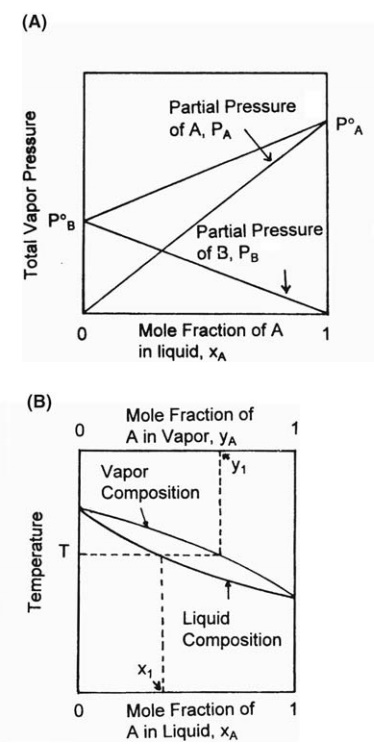
FIGURE 10.4 (A) The vapor pressure of an
ideal binary mixture. (B) Phase diagram.
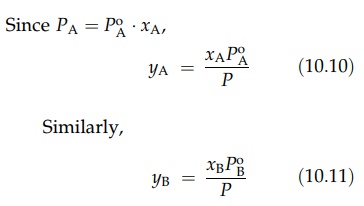
If
A is the more volatile component, PAo is greater than P ·
yA is therefore greater than xA, that is, the vapor is
richer in the more volatile component than the liquid with which it is in
equilibrium.
The Relation of Boiling Point
and Mixture Composition
For
the purposes of distillation, curves relating vapor pressure and composition
are usually replaced by boiling point curves. These are determined by
experi-ment at the given pressure. Figure 10.5A represents a system in which
the vapor pressure of some mixtures is greater than the vapor pressure of the
pure, more volatile component. This system will exhibit a minimum boiling
point, and the composition of the liquid at this point is given by Z. This
mixture, which is a constant-boiling or azeotropic mixture, evolves on boiling
a vapor of the same composition. In the binary system described in Figure
10.5B, mixtures are formed with a vapor pressure that is less than that of the
less volatile compo-nent. The maximum boiling point is given by the azeotropic
mixture, Z.
Systems
that form minimum-boiling mixtures are common. Ethyl alcohol and water provide
an example, the azeotrope containing 4.5% by weight of water. The boiling point
at atmospheric pressure is 351.15 K, 0.25 K lower than the boiling point of
pure alcohol. Maximum-boiling mixtures are less common. The most familiar example
is hydrochloric acid, which forms an azeotrope boiling at 381 K and contains
20.2% by weight of hydrochloric acid.
Mixtures
that form azeotropes cannot be separated into the pure compo-nents by normal
distillation methods. However, separation into the azeotrope and one pure
component is possible. Efficient fractionation of the mixture M of Figure 10.5A
would give the azeotrope Z as distillate and pure B as the residue.
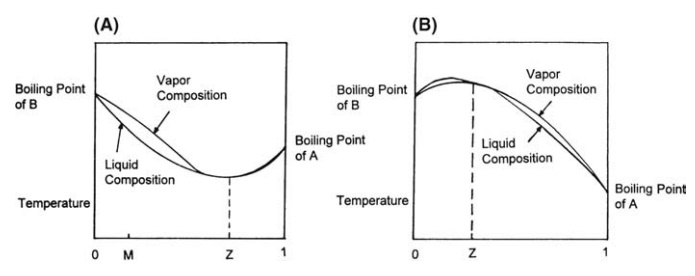
FIGURE 10.5 Temperature-composition diagrams
for a binary mixture. (A) Minimum azeotrope and (B) maximum azeotrope.
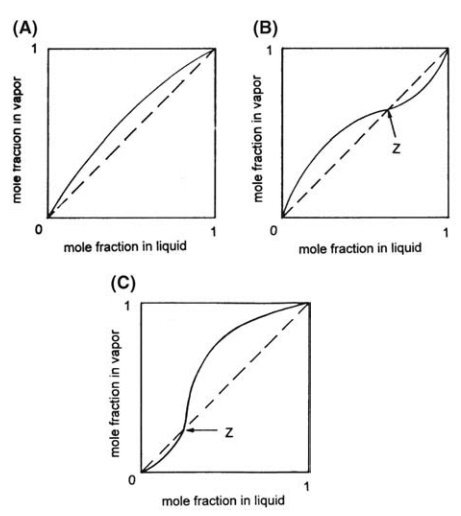
FIGURE 10.6 Vapor-liquid equilibrium diagrams.
The
composition of the azeotropic mixture of a system is a function of the total
pressure, and it is possible, in some cases, to eliminate the constant-boiling
mixture by altering the pressure at which the distillation is performed. For
example, at pressures less than 100 mmHg, ethyl alcohol and water do not form
an azeotrope. At this pressure, they can be completely separated.
Vapor-Liquid Equilibrium
Diagrams
Vapor-liquid
equilibrium diagrams of the form shown in Figure 10.6 provide an alternative
and convenient method of recording distillation data. They consist of a
conventional graph relating the mole fraction of the more volatile component in
the liquid, designated X, to the mole fraction of the more volatile component
in the vapor, designated Y. An ideal binary system is shown in Figure 10.6A.
The temperature varies along each of the curves, and the diagram is only
applicable to the pressure at which the variables were measured. Curves of
min-imum-boiling mixtures and maximum-boiling mixtures are drawn in Figure
10.6B and C, respectively.
Simple or Differential Distillation
In
simple or differential distillation, the vapor evolved from the boiling mixture
is immediately removed and condensed. For the system shown in Figure 10.7A, the
liquid of composition x1 evolves a vapor of composition y1.
Its removal impoverishes the liquid in the more volatile component. The
composition of the liquid moves toward pure B, and its boiling point increases.
There is, therefore, a progressive change in the composition of the vapor, the
mole fraction of the more volatile component steadily decreasing. Unless the
boiling points of the two pure components differ widely, a reasonable degree of
separation is not possible. The method may be used to remove low–boiling point
solvents from aqueous solutions.
Related Topics
