Binary Mixtures of Immiscible Liquids: Steam Distillation
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Evaporation and Distillation
Distillation is a process in which a liquid mixture is separated into its component parts by vaporization.
DISTILLATION
Distillation
is a process in which a liquid mixture is separated into its component parts
by vaporization. The vapor evolved from a boiling liquid mixture is normally
richer in the more volatile components than the liquid with which it is in
equilibrium. Distillation rests on this fact. Although multicomponent mix-tures
are most common in distillation processes, an understanding of the operation
can be based on the vapor pressure characteristics of two-component or binary
mixtures. Binary systems in which the liquids are immiscible are discussed
first. Discussion of the separation of miscible liquids by fractionation forms
most of the remainder of the section.
Binary Mixtures of Immiscible Liquids: Steam Distillation
If
the two components of a binary mixture are immiscible, the vapor pressure of
the mixture is the sum of the vapor pressures of the two components, each
exerted independently and not as a function of their relative concentrations in
the liquid. This property is employed in steam distillation, a process
particularly applicable to the separation of high–boiling point substances from
nonvolatile impurities. The steam forms a cheap and inert carrier. The
principles of the process, however, apply to other immiscible systems.
If
a mixture of water and a high–boiling point liquid, such as nitrobenzene, is
heated, the total vapor pressure increases and ultimately reaches the external
pressure. The mixture boils, and the vapors evolved are condensed to give a
liquid mixture, which separates under gravity. In practice, the vapors are
pro-duced by blowing steam into the liquid in a manner that gives intimate
contact between the phases. Since both components contribute to the total
pressure, the boiling temperature must be lower than the boiling point of
either component. In the case of nitrobenzene and water, the boiling point at
atmospheric pressure is about 372 K. To distill nitrobenzene alone at this
temperature, a pressure of 20 mmHg must be imposed. Steam distillation,
therefore, permits the distillation of water-immiscible materials of high
boiling point without the use of high temperatures, which might cause
decomposition, or high vacua. The method, however, will only separate such
materials from nonvolatile constituents. If volatile impurities are present,
these will appear in the distillate.
The
composition of the distillate is calculated in the following way. For two
components, A and B, the total vapor pressure, P, is the sum of the vapor
pressures of the components, PA and PB. Since the partial
pressure of a com-ponent in a gaseous mixture is proportional to its molar
concentration, the composition of the vapor is given by

where
nA and nB are the number of moles of A and B in the
vapor, respectively. If WA and WB are the weights of A
and B in the vapor, then
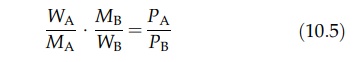
where MA and MB are the respective molecular weights. The distillate obtained from the vapor is WA + WB. Therefore,
Percentage
of A in the distillate = WA / (WA + WB) x 100 = PAMA / (PAMA + PBMB) x 100 (10:6)
The
ratio of immiscible organic liquid to water in the distillate is increased if
the former has a high molecular weight or a high vapor pressure.
Steam
distillation under vacuum may be employed when the thermal stability of the
material prohibits temperatures of about 373 K. A further variant is the
introduction of unsaturated steam under conditions in which no con-densation to
water takes place. Only two phases, the liquid being distilled and the mixed
vapors, are then present. The external pressure no longer fixes the
temperature, as in a three-phase system, and any convenient value can be
chosen.
The
chief uses of steam distillation are the purification and isolation of liquids
of high boiling point, such as aniline, nitrobenzene, or s-dichlorobenzene, and the
preparation of fatty acids and volatile oils. Many of the latter are pre-pared
by introducing steam into a mixture of the comminuted drug and water. The
method is also used to remove odoriferous elements, such as aldehydes and
ketones, from edible oils. The dehydration of a material by adding a volatile,
water-immiscible solvent, such as toluene, and distilling the mixture is a form
of steam distillation. The solvent separates in the condensate and may be
returned to the still.
Related Topics
