Crystallizers
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Crystallization
Although other methods may be adopted, crystallizers can be conveniently classified by the way in which a solution is supersaturated.
CRYSTALLIZERS
Although
other methods may be adopted, crystallizers can be conveniently classified by
the way in which a solution is supersaturated. This leads to the
self-explanatory terms: cooling crystallizer and evaporative crystallizer. In
vacuum crystallizers, both evaporation and cooling are used.
Cooling Crystallizers
Open
or closed tanks, agitated by stirrers, are used for batch crystallization. The
specific heat of the solution and the heat of crystallization are removed by
means of jackets or coils through which cooling water can be circulated.
Agitation destroys temperature gradients in the tanks, opposes sedimentation
and the irregular growth of crystals at the bottom of the vessel, and, as
described above, facilitates growth. Similar equipment is used for
crystallization or precipitation by the addition of a third substance.
Crystallizers
for continuous processes often take the form of a trough cooled naturally or by
a jacket. The solution enters at one end, and crystals and liquid are
discharged at the other. In one type of crystallizer, a slow-moving worm works
in the solution and lifts crystals off the cooling surface to shower them
through the solution and slowly convey them through the trough. The trough of
another is agitated by rocking. Baffles are used to increase the resi-dence
time of the solution. Both crystallizers are characterized by low heat transfer
coefficients, and an alternative arrangement consists essentially of a
double-pipe heat exchanger. The crystallizing fluid is carried in the central
pipe with countercurrent flow of the coolant in the annulus between the pipes.
A shaft rotates in the central tube carrying blades, which scrape the heat
transfer surface. High heat transfer coefficients are obtained. An Oslo
crystallizer, in which supersaturation is given by cooling, is described in
Figure 9.6A. The principles underlying this plant have already been described.
Evaporative Crystallizers
On
a small scale, simple pans and stirred reaction vessels can be used for
evaporative crystallization. Larger units may employ calandria heating, as
shown in Figure 9.6B. The downcomer, which must be large enough to accommodate
the flow of the suspension, commonly houses an impeller, forced circulation
increasing the heat transfer to the boiling/liquid. These units may be adapted
for either batch or continuous processes in which crystal size is not of great
importance. For continuous processes demanding close control of product size,
an Oslo crystallizer, which saturates the solution by evaporation, may be
employed.
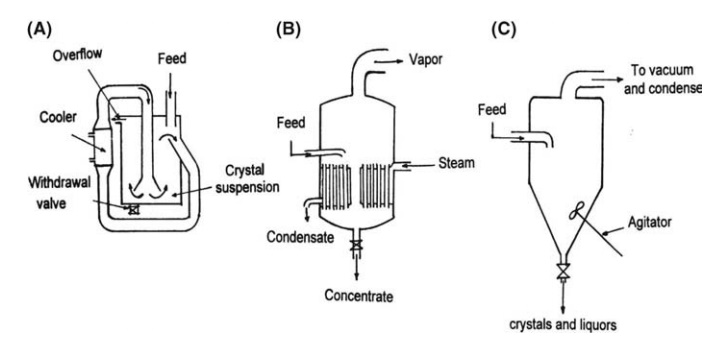
FIGURE 9.6 (A) Cooling crystallizer, (B)
evaporative crystallizer, and (C) batch vacuum crys-tallizer.
Vacuum Crystallizers
Vacuum
crystallizers produce supersaturated conditions by solvent removal and cooling.
An example is shown in Figure 9.6C. A hot, concentrated solution is fed to an agitated
crystallization chamber maintained at low pressure. The solution boils and
cools adiabatically to the boiling point corresponding to the operating
pressure. Crystallization follows concentration, and the product is removed
from the bottom of the vessel. The principles of the Oslo crystallizers are
also employed in vacuum crystallization.
Related Topics
