Crystallization from Solutions
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Crystallization
When a material crystallizes from a solution, nucleation and crystal growth occur simultaneously over a wide intermediate temperature range so that a study of these processes is more difficult.
CRYSTALLIZATION FROM SOLUTIONS
When a material
crystallizes from a solution, nucleation and crystal growth occur
simultaneously over a wide intermediate temperature range so that a study of
these processes is more difficult. In general, however, they are thought to be
similar to nucleation and crystal growth in melts. The three basic steps,
induction of supersaturation, formation of nuclei, and growth of crystals, are
explained with reference to the solubility curve shown in Figure 9.2.
A
solution with temperature and concentration indicated by point A may be
saturated by either cooling to point B or removing solvent (point C). With
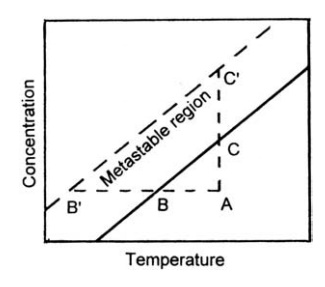
FIGURE 9.2 The solubility-supersolubility
diagram.
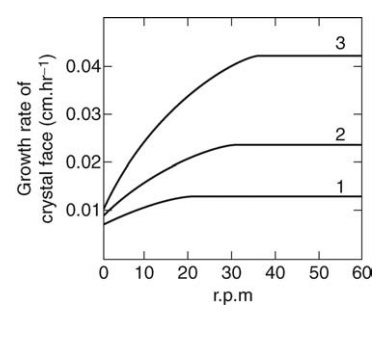
FIGURE 9.3 The effect of agitation on the rate of growth of a crystal of sodium thiosulfate.
If the degree
of supersaturation is small, the spontaneous formation of crystal nuclei is
highly improbable. Crystal growth, however, can occur if seeds are added. With
greater supersaturation, spontaneous nucleation becomes more probable and the
metastable region will be limited approximately by the line B’ C’ . If the solution
is cooled to B’
or concentrated by solvent removal to C’ , spontaneous nucleation is
virtually certain. Crystal growth will also occur in these conditions. The rate
of growth, however, is depressed at low temperatures.
During crystal
growth, deposition on the faces of the crystal causes depletion of molecules in
the immediate vicinity. The driving force is provided by the concentration
gradient setup, from supersaturation in the solution to lower concentrations at
the crystal face. A large degree of supersaturation, therefore, promotes a high
growth rate. A reaction at the surface, in which solute molecules become
correctly orientated in the crystal lattice, provides a second resistance to
the growth of the crystal. Simultaneously, the heat of crystallization must be
conducted away.
Agitation modifies
the rate of crystal growth for given conditions of temperature and saturation.
Initially, agitation quickly increases the rate of growth by decreasing the
thickness of the boundary layer and the diffusional resistance. However, as
agitation is intensified, a limiting value is reached, which is determined by
the kinetics of the surface reaction. In Figure 9.3, the effect of agitation on
the rate of crystal growth in solutions of sodium thiosulfate of differing
degrees of supersaturation is described.
As with melts,
soluble impurities may increase or retard the rate of nucleation. Insoluble
materials may act as nuclei and promote crystallization. Impurities may also
affect crystal form and, in some cases, are deliberately added to secure a
product with good appearance, absence of caking, or suitable flow properties.
The temperature at
which crystallization is performed may be determined by the crystal form or
degree of hydration required of the products. Reference to the solubility
curves given in Figure 9.4 shows that crystallization at 50○C yields FeSO4
· 7H2O, at 60○C
yields FeSO4N · 4H2O, and at 70○C, FeSO4.
The majority of materials, however, have one or possibly two forms. The degree
of super-saturation of solution 1 is 5 g/L, of solution 2 is 10 g/L, and of
solution 3 is 15 g/L.
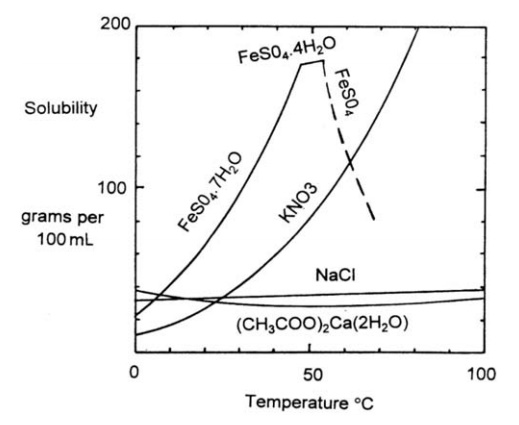
FIGURE 9.4 Solubility curves.
Related Topics
