Production of Large Crystals
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Crystallization
Batch production of large, uniform crystals may be carried out in agitated reaction vessels by slow, controlled or natural cooling. Spontaneous nucleation is improbable until solution A is cooled to X.
PRODUCTION OF LARGE CRYSTALS
Batch
production of large, uniform crystals may be carried out in agitated reaction
vessels by slow, controlled or natural cooling. Spontaneous nucleation is
improbable until solution A is cooled to X. Crystallization then follows the
path XB. Better control is gained if the solution is artificially seeded.
Seeding is shown at X’
. Crystallization then follows the broken line X’ B, the aim being to
maintain the solution in the metastable region where growth rate is high and
natural nucleation is low. The course of the crystallization is shown in Figure
9.5. Initially, spontaneous nucleation may be allowed by cooling from A to X.
As crystallization takes place, the degree of supersaturation and the
concentration of the solute fall, ultimately reaching saturation at B when
growth will cease. Closer control is secured by artificially seeding the
supersaturated solution in
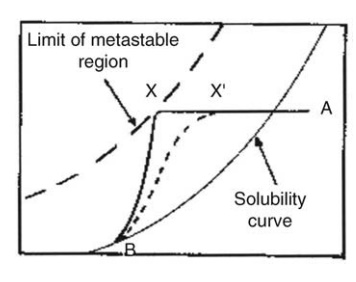
FIGURE 9.5 The production of large
crystals. The conditions of supersaturation.
An
important principle for the continuous production of large even crystals is
used in Oslo or Krystal crystallizers. A metastable, supersaturated solution is
released into the bottom of a mass of growing crystals on which the solute is
deposited. The crystals are fluidized by the circulation of the solution, and
classification in this zone allows the withdrawal of sufficiently large
crystals from the bottom.
Related Topics
